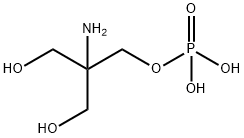
Fosfomycin Trometamol impurity C synthesis
- Product Name:Fosfomycin Trometamol impurity C
- CAS Number:23001-39-0
- Molecular formula:C4H12NO6P
- Molecular Weight:201.11
![Carbamic acid, N-[5-[[[bis(phenylmethoxy)phosphinyl]oxy]methyl]-2,2-dimethyl-1,3-dioxan-5-yl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1404217-43-1.gif)
1404217-43-1
0 suppliers
inquiry

23001-39-0
49 suppliers
inquiry
Yield: 76%
Reaction Conditions:
Stage #1:tert-butyl N-[5-({[bis(benzyloxy)phosphoryl]oxy}methyl)-2,2-dimethyl-1,3-dioxan-5-yl]carbamate with hydrogen;5%-palladium/activated carbon in methanol for 2 h;
Stage #2: with trifluoroacetic acid in water for 0.5 h;
Steps:
30
Scheme 30Dibenzyl diisopropyl phoshoramidite (3.64 mL, 10.83 mmol) is added to an ice cold solution of terf-butyl /V-[5-(hydroxymethyl)-2,2-dimethyl-1 ,3-dioxan-5-yl]carbamate (147) [Ooi, H.; Ishibashi, N.; Iwabuchi, Y.; Ishihara, J. Hatakeyama, S., J. Org. Chem. 2004, 69, 7765-7768.] (1.35g, 5.42 mmol) and 1H-tetrazole (1.52 g, 21.7 mmol) in CH2CI2 (50 mL). The solution is stirred at room temperature for 1 h and then cooled to -30 °C. A solution of MCPBA (4.67g, 16.3 mmol) in CH2CI2 (25 mL) is added and the solution bought slowly to room temperature. The solution is partitioned between CH2CI2 and 0% aqueous sodium bicarbonate. The organic phase is dried and concentrated under reduced pressure. The residue is flash chromatographed (CH2CI2-MeOH, 9:1) to give crude terf-butyl A/-[5-(hydroxymethyl)-2,2-dimethyl-1 ,3-dioxan-5-yl]carbamate (148) (2.81 g, 99%). A portion of compound (148) (0.2 g) is further flash chromatographed (EtOAc- hexanes) and the purified product is then dissolved in methanol (40 mL) and stirred with palladium on charcoal (5%, 22 mg) under a balloon of hydrogen. After 2 h the catalyst is removed by filtration. The solution is concentrated to dryness and the residue dissolved in 90% aqueous TFA. After 30 min the solution is concentrated under reduced pressure and the residue dissolved in H20. Lyophilization gives [2-amino-3-hydroxy-2- (hydroxymethyl)propoxy]phosphonic acid (149) (90 mg, 0.29 mmol, 76%). 1H NMR (500 MHz, D20, HOD 4.70 ppm) δ 3.99 (s, 2H), 3.75 (s, 4H). 13C NMR (125.7 MHz, D20, referenced to internal acetone at δ 30.3) δ 62.8 (d, Jc,p = 4.6 Hz), 60.8 (d, Jc,p = 8.1 Hz), 59.3. 31P NMR (202.4, MHz, D20) δ 0.0. ESI-HRMS C4H13N06P [M+H]+calcd. 202.0481 , found 202.0484.
References:
INDUSTRIAL RESEARCH LIMITED;ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIVERSITY;CLINCH, Keith;CRUMP, Douglas Ronald;EVANS, Gary Brian;HAZLETON, Keith Zachary;MASON, Jennifer Mary;SCHRAMM, Vern L.;TYLER, Peter Charles WO2012/150866, 2012, A1 Location in patent:Page/Page column 101-102