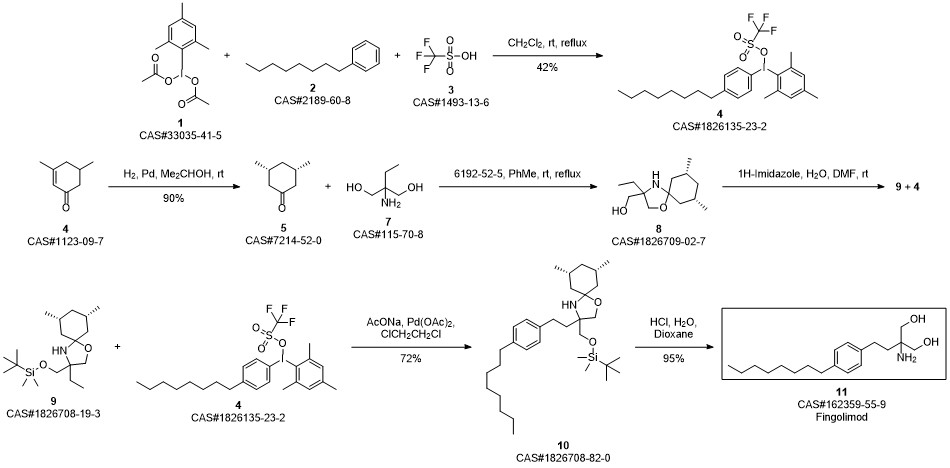
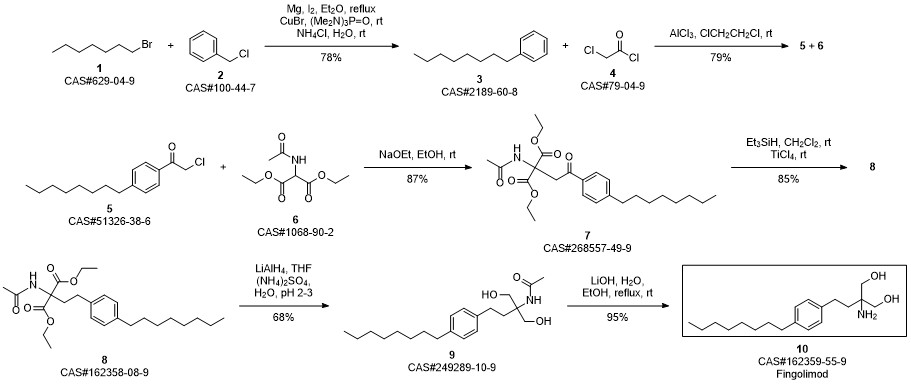
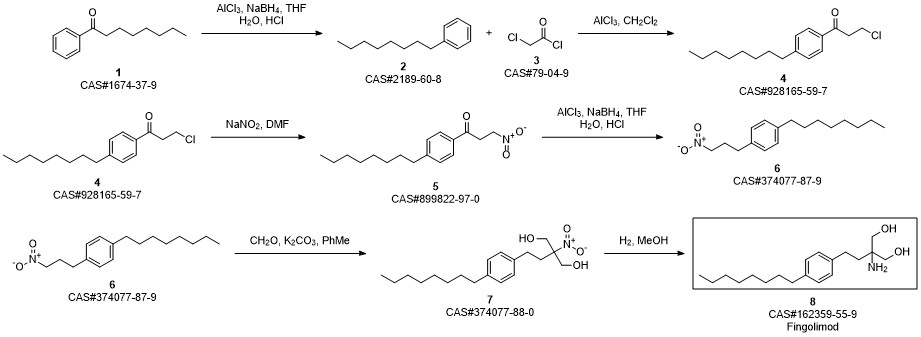
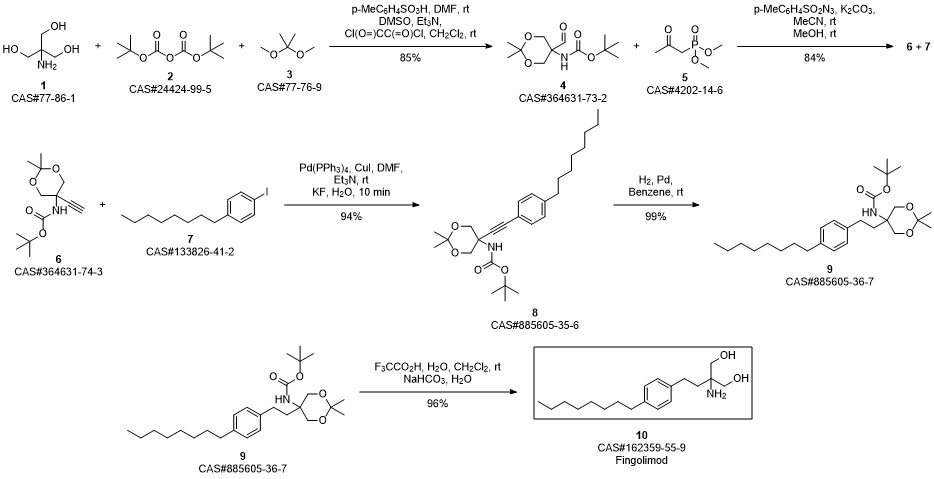
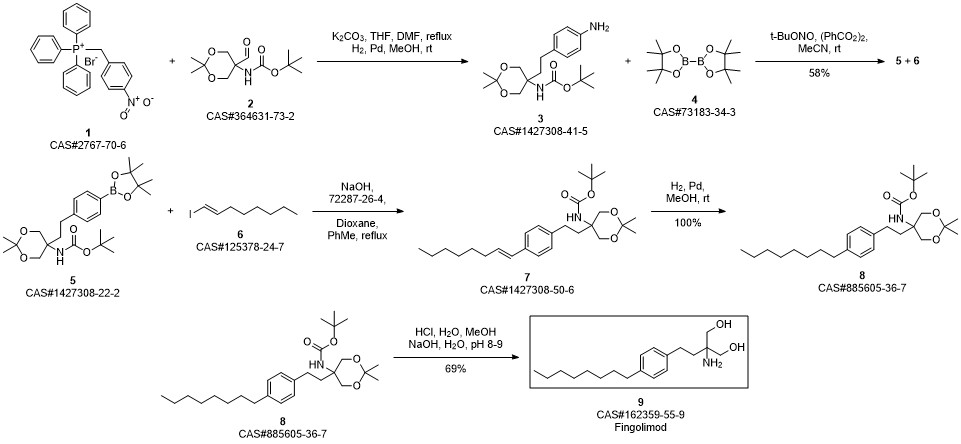
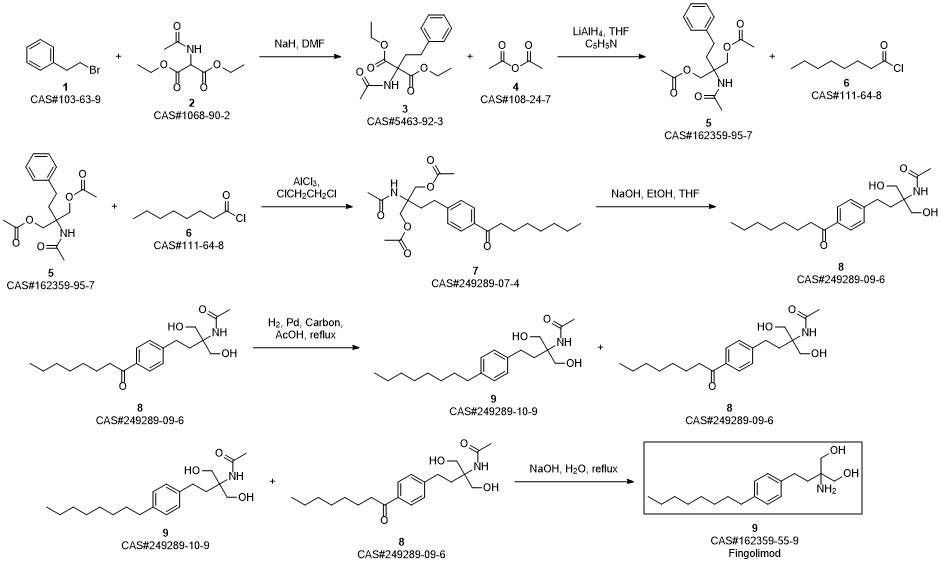
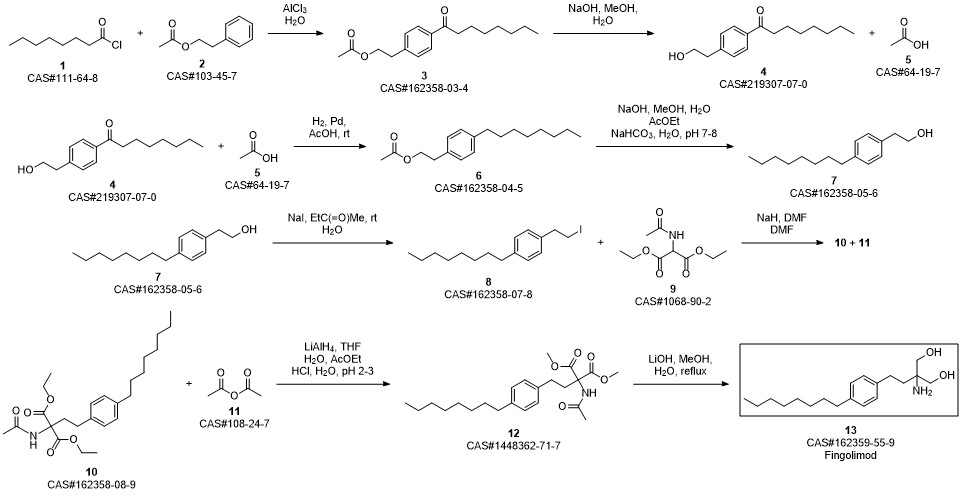
Gilenia synthesis
- Product Name:Gilenia
- CAS Number:162359-55-9
- Molecular formula:C19H33NO2
- Molecular Weight:307.47
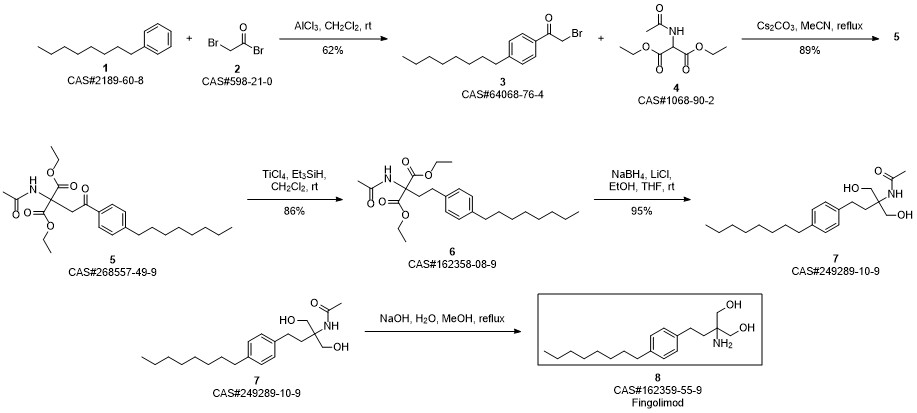
Shaikh, Rizwan S.; Schilson, Stefanie S.; Wagner, Stefan; Hermann, Sven; Keul, Petra; Levkau, Bodo; Schaefers, Michael; Haufe, Guenter. Synthesis and Evaluation of Fluorinated Fingolimod (FTY720) Analogues for Sphingosine-1-Phosphate Receptor Molecular Imaging by Positron Emission Tomography. Journal of Medicinal Chemistry. Volume 58. Issue 8. Pages 3471-3484. Journal; Online Computer File. (2015).
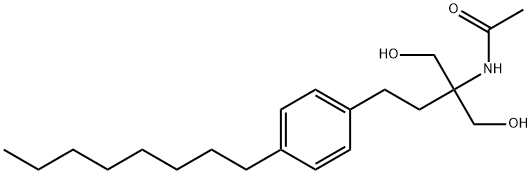
249289-10-9
95 suppliers
$105.00/50mg

162359-55-9
176 suppliers
$7.00/5mg
Yield:162359-55-9 82%
Reaction Conditions:
with sodium hydroxide in methanol for 5 h;Reflux;
Steps:
12.5 12.5 2-Amino-2-(4-octylphenethyl)propane-1 ,3-diol (FTY 720) (SSS 798)
Protected aminodiol 71 (349 mg, 1.0 mmol) was dissolved in methanol (20 mL) and treated with 1 M sodium hydroxide solution (1.2 mL, 1.2 mmol, 1.2 eq.). The reaction mixture was heated to reflux for 5 h. After cooling to r.t. the mixture was diluted with 1 M sodium hydroxide solution (15 mL) and the aqueous phase was extracted with dichloromethane (5 x 20 mL). The combined organic layers were dried over Na2S04 and concentrated in vacuo. The product was crystallized from ethyl acetate to give a white solid. Yield: 252 mg (82%). 72 M.p.: 127 °C (lit. 121 -124 °C) 1H-NMR (300 MHz, CD3OD, CDCI3) δ [ppm]: 0.87 (m, 3 H, 19-CH3), 1 .22-1.37 (m, 10 H, 14-CH2 to I 8-CH2), 1 .57 (m, 2 H, 13-CH2), 1 .69 (m, 2 H, 4-CH2), 2.51 -2.65 (m, 4 H, 5-CH2, 12-CHz), 3.48 (d, 2JH,H = 10.9 Hz, 2 H, 1 -CH2, 3-CH2), 3.54 (d, 2JH,H = 1 1.0 Hz, 2 H, 1 -CH2, 3-CHz), 7.09 (m, 4 H, 7-CH, 8-CH, 10-CH, 1 1 -CH). 13C-NMR (75 MHz, CD3OD, CDCI3) δ [ppm]: 14.3 (q, C-19), 23.2 (t, C-18), 29.5, 29.9, 30.1 (t, C-13 to C-16), 32.2 (t, C-5), 32.5 (t, C-4), 36.1 (t, C-17), 37.0 (t, C-12), 56.4 (s, C-2), 66.2 (t, C-1 , C-3), 128.7, 129.0 (d, C-7, C-8, C-10, C-1 1 ), 140.1 , 140.9 (s, C-6, C-9). Exact mass (ESI+): C19H33NO2 + H+: calcd. 308.2584, found 308.2585; C19H33NO2 + Na+: calcd. 330.2404, found 330.2408; + Na+: calcd. 637.4915, found 637.4917. Ref.: Spectroscopic data agree with those given in S. Kim, H. Lee, M. Lee, T. Lee, Synthesis 2006, 5, 753-755.
References:
WESTFAELISCHE WILHELMS-UNIVERSITAET MUENSTER;HAUFE, Guenter;LEVKAU, Bodo;SCHAEFERS, Michael;SCHILSON, Stefani Silke;KEUL, Petra WO2013/26765, 2013, A1 Location in patent:Page/Page column 66-67
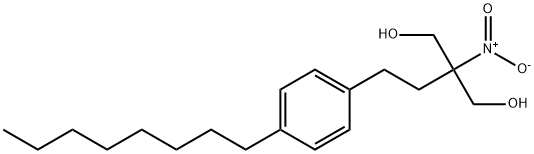
374077-88-0
35 suppliers
inquiry

162359-55-9
176 suppliers
$7.00/5mg
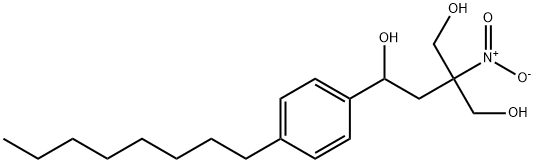
899822-99-2
55 suppliers
inquiry

162359-55-9
176 suppliers
$7.00/5mg
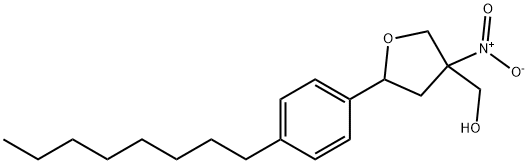
1369968-69-3
0 suppliers
inquiry

162359-55-9
176 suppliers
$7.00/5mg
![N-[2,2-DiMethyl-5-[2-(4-octylphenyl)ethyl]-1,3-dioxan-5-yl]carbaMic acid 1,1-diMethylethyl ester](/CAS/20150408/GIF/885605-36-7.gif)
885605-36-7
4 suppliers
inquiry

162359-55-9
176 suppliers
$7.00/5mg