
Glutaraldehyde synthesis
- Product Name:Glutaraldehyde
- CAS Number:111-30-8
- Molecular formula:C5H8O2
- Molecular Weight:100.12

285-67-6
251 suppliers
$30.00/5g

111-30-8
521 suppliers
$13.50/25ML
Yield:111-30-8 99 %Chromat.
Reaction Conditions:
with 1-(3-sulfopropyl)-2-methyl-3-isopropylimidazolium periodate in water at 35; for 12 h;
Steps:
10
4.40 g (0.01 mol) of an acidic ionic liquid of 1-sulfopropyl-2-methyl-3-isopropylimidazolium iodide ([HSO 3 -PMPIM] IO 4) dissolved in deionized water was added to a single port The flask was magnetically stirred for 0.5 hour. Followed by addition of 0.84 g (0.01 mol) of epoxyclopentane, temperature 35 ° C, and magnetic stirring for 12 hours. To obtain a transparent liquid with a white precipitate in the lower layer. Centrifugal separation, take the supernatant clear liquid, that is, the target product of glutaraldehyde. The lower white precipitate was washed alternately with ethanol and water for 3-4 times, followed by electrolytic oxidation of 1-sulfopropyl-2-methyl-3-isopropylimidazolium iodide ([HSO 3 -PMPIM] IO 3) is oxidized to 1-sulfopropyl-2-methyl-3-isopropylimidazolium iodide ([HSO 3 -PMPIM] IO 4) ionic liquid and then applied repeatedly. The yield of glutaraldehyde was determined to be 99% by GC.
References:
CN106279035,2017,A Location in patent:Paragraph 0138; 0139
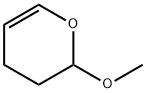
4454-05-1
142 suppliers
$9.00/25g

111-30-8
521 suppliers
$13.50/25ML

4454-02-8
1 suppliers
inquiry

111-30-8
521 suppliers
$13.50/25ML
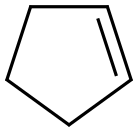
142-29-0
259 suppliers
$14.00/25mL

111-30-8
521 suppliers
$13.50/25ML

142-29-0
259 suppliers
$14.00/25mL
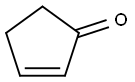
930-30-3
268 suppliers
$10.00/1g

111-30-8
521 suppliers
$13.50/25ML