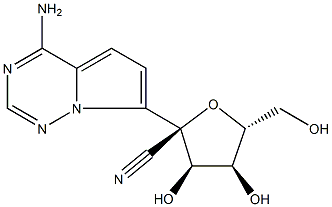
GS-441524 synthesis
- Product Name:GS-441524
- CAS Number:1191237-69-0
- Molecular formula:C12H13N5O4
- Molecular Weight:291.26
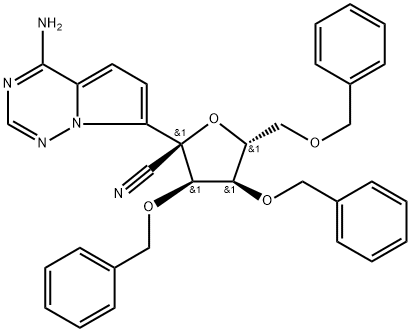
1355357-49-1
126 suppliers
inquiry

1191237-69-0
345 suppliers
$8.00/1mg
Yield:1191237-69-0 95%
Reaction Conditions:
with boron trichloride in dichloromethane at 5;
Steps:
1
(1) Material preparation: Dissolve about 200g of raw materials in dichloromethane to prepare 0.5mmol/mL raw material liquid, and place it in the raw material liquid bottle;1.0mmol/mL boron trichloride dichloromethane solution (about 1200mL) is directly used as the auxiliary material liquid and placed in the auxiliary material liquid bottle;Connect the raw material liquid bottle to the metering pump A;Connect the auxiliary material liquid bottle to the metering pump B;(2) Reaction temperature parameter setting: start the circulating liquid cooling system, make the mixer and the main reaction pipeline at about 5 degrees of the set temperature;(3) Material flow rate parameter setting: the flow rate parameter of metering pump A is set to 25mL/min (12.5mmol/min); the flow rate parameter of metering pump B is set to 37.5mL/min (37.5mmol/min);(4) Continuous flow reaction system operation: start two pump systems at the same time, metering pump A and metering pump B respectively send the raw material liquid and auxiliary material liquid into the mixer at their respective set flow rates; the two feed liquids are mixed in the pre-cooling mixer (Holding liquid retention time is about 12 seconds), and then enters the pre-cooling main reaction pipeline (Holding liquid retention time is about 50 seconds);(5) Quenching process: the reaction liquid flows out into an aqueous sodium hydroxide solution (120g sodium hydroxide, dissolved in 1.8kg water) for quenching;(6) Post-treatment process: filtering, adding the obtained crude product to 150 g of methanol, beating and filtering to obtain 98.5 g of the target product, with a yield of 95%, a purity of the high performance liquid phase of 99.4%, and a single impurity of less than 0.15%.
References:
CN112745324,2021,A Location in patent:Paragraph 0040-0048
![(3R,4R,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-bis(benzyloxy)-5-((benzyloxy)methyl)tetrahydrofuran-2-carbonitrile](/CAS/20211123/GIF/1191237-68-9.gif)
1191237-68-9
5 suppliers
inquiry

1191237-69-0
345 suppliers
$8.00/1mg
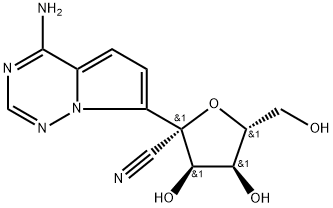
1355049-95-4
28 suppliers
inquiry
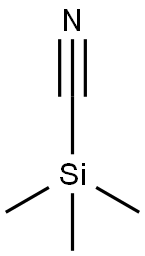
7677-24-9
395 suppliers
$19.00/5g
![D-Ribitol, 1-C-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-1,4-anhydro-2,3,5-tris-O-(phenylmethyl)-, (1S)-](/CAS/20210111/GIF/158227-80-6.gif)
158227-80-6
3 suppliers
inquiry

1191237-69-0
345 suppliers
$8.00/1mg
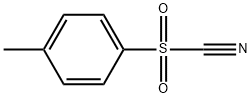
19158-51-1
177 suppliers
$52.00/1g
![D-Ribitol, 1-C-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-1,4-anhydro-2,3,5-tris-O-(phenylmethyl)-, (1S)-](/CAS/20210111/GIF/158227-80-6.gif)
158227-80-6
3 suppliers
inquiry

1191237-69-0
345 suppliers
$8.00/1mg
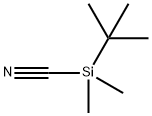
56522-24-8
37 suppliers
$25.00/1g
![D-Ribitol, 1-C-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-1,4-anhydro-2,3,5-tris-O-(phenylmethyl)-, (1S)-](/CAS/20210111/GIF/158227-80-6.gif)
158227-80-6
3 suppliers
inquiry

1191237-69-0
345 suppliers
$8.00/1mg