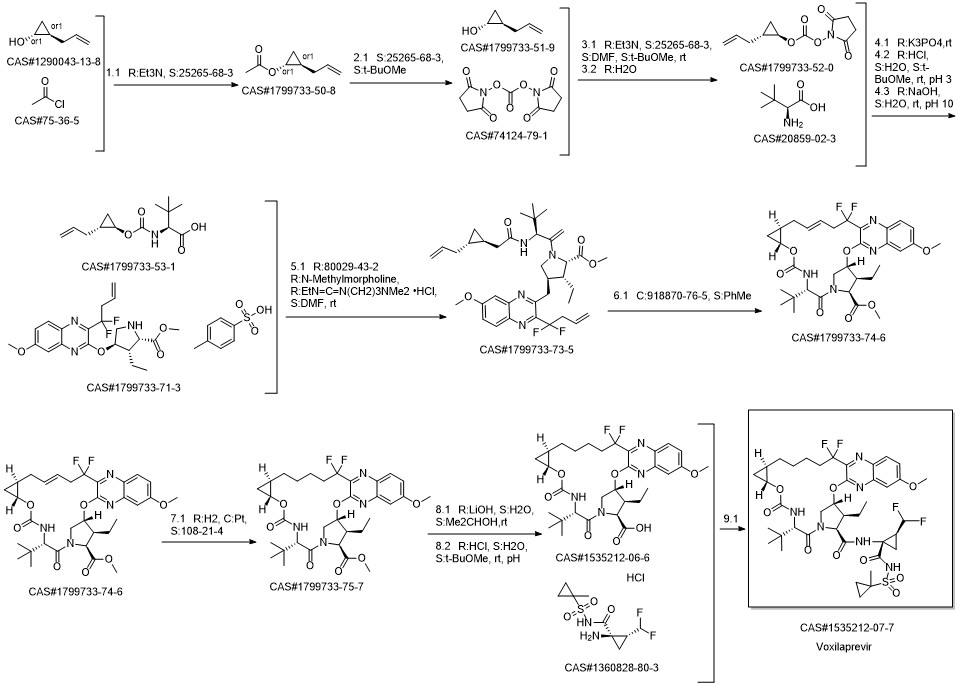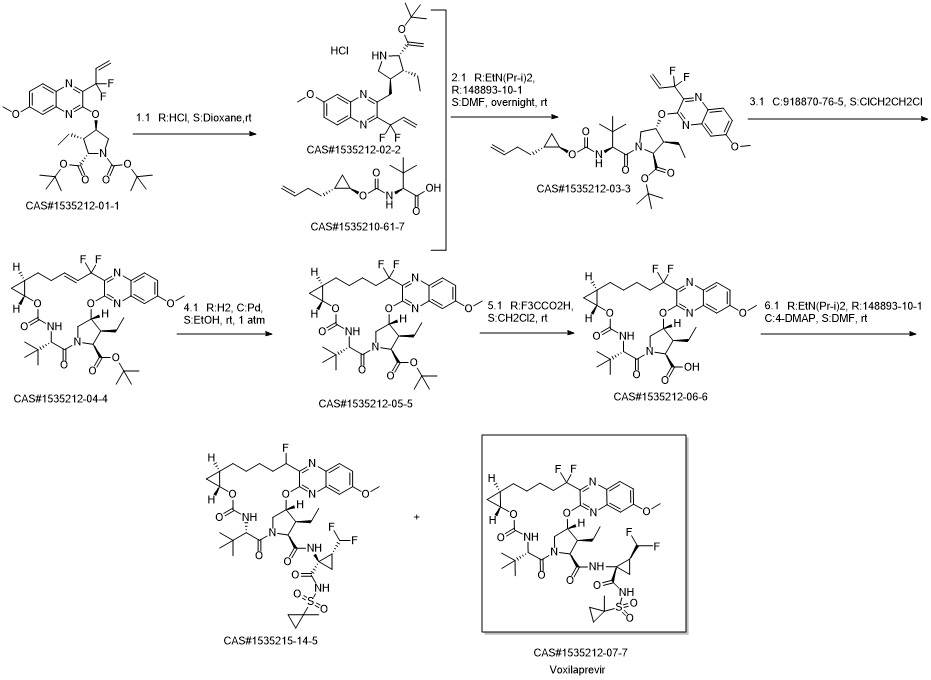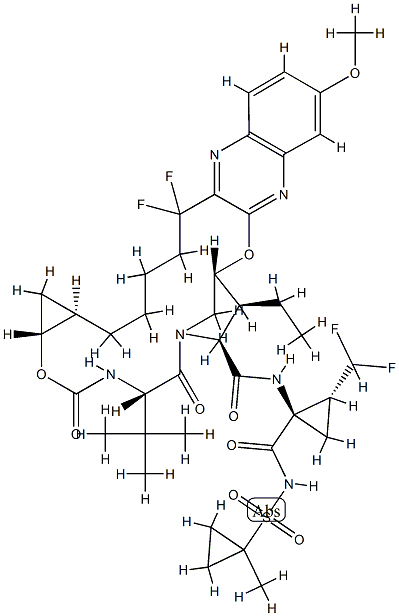
GS9857 synthesis
- Product Name:GS9857
- CAS Number:1535212-07-7
- Molecular formula:C40H52F4N6O9S
- Molecular Weight:868.93
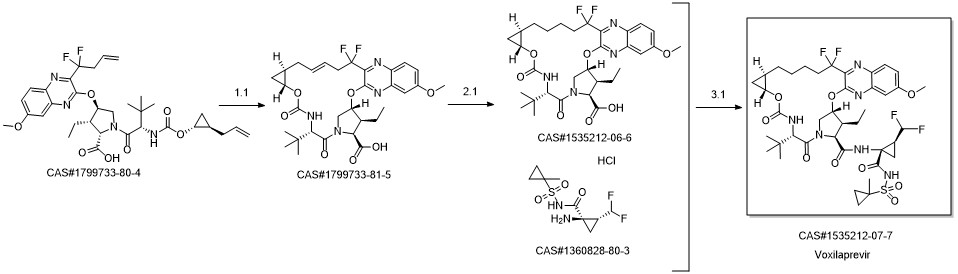
Reference: Cagulada, Amy; Chan, Johann; Chan, Lina; Colby, Denise A.; Karki, Kapil Kumar; Kato, Darryl; Keaton, Katie Ann; Kondapally, Sudha; Levins, Chris; Littke, Adam; Martinez, Ruben; Pcion, Dominika; Reynolds, Troy; Ross, Bruce; Sangi, Michael; Schrier, Adam J.; Seng, Pamela; Siegel, Dustin; Shapiro, Nathan; Tang, Donald; Taylor, James G.; Tripp, Jonathan; Waltman, Andrew W.; Yu, Lawrence. Synthesis of an antiviral N-(3-ethyl)prolyl-1-aminocyclopropanecarboxylic acid peptide and new routes to its difluoromethylaminocyclopropanecarboxylic acid intermediate. Assignee Gilead Sciences, Inc., USA. US 20150175626. (2015).
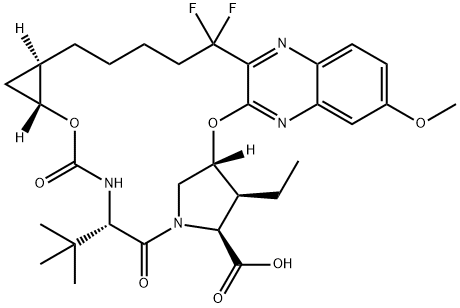
1535212-06-6
5 suppliers
inquiry
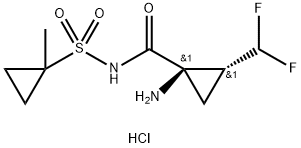
1360828-80-3
66 suppliers
inquiry

1535212-07-7
43 suppliers
inquiry
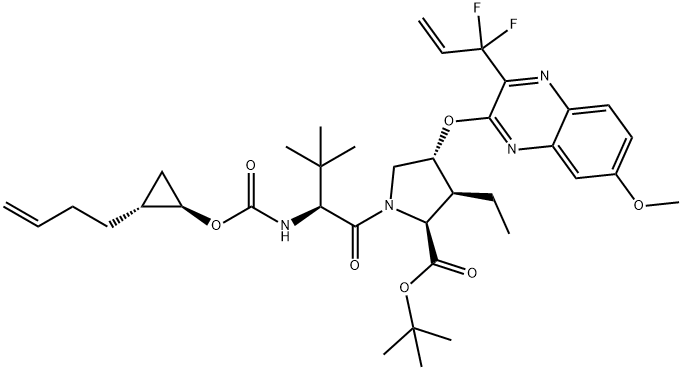
Yield:1535212-07-7 95 mg
Reaction Conditions:
with dmap;N-ethyl-N,N-diisopropylamine;HATU in N,N-dimethyl-formamide at 20; for 0.666667 h;
Steps:
17.7 Step 7. Preparation of Example 17:
Step 7. Preparation of Example 17: A mixture of carboxylic acid 17-6 (153 mg, 0.247 mmol), Intermediate AlO (90 mg, 0.297 mmol), HATU (113 mg, 0.297mmol), DMAP (45 mg, 0.37 mmol) and DIPEA (0.215 mL, 1 .24 mmol) in DMF (1 .5 mL) was stirred at rt for 40 minutes. The mixture was diluted with 2 N aqueous HCI (2 mL) and extracted with dichioromethane. The organic phase was dried over sodium sulfate, filtered and concentrated. The crude product mixture was purified by silica gel chromatography (EtOAc in hexanes: 30% - 95%) to give Example 17 (95 mg). Analytic HPLC RetTime: 8.79 mi LCMS-ESI(m/z): [M+H]calcd for C40H53F4N609S: 869.3; found: 869.2. 1H NMR (400 MHz,ODd3) 6 9.948 (br s, 1 H), 7.99 (d, J = 9.2 Hz, 1 H), 7.29 (dd, J = 8.8, 2.4 Hz, 1 H),7.09 (d, J = 2.8 Hz, 1 H), 6.57 (br s, 1 H), 5.97 (td, JH-F = 52 Hz, J = 6.8 Hz, 1 H),5.92 (d, J = 3.6 Hz, 1 H), 5.322 (d, J = 9.6 Hz, 1 H), 4.42 (ap d, J = 7.2 Hz, 1 H),4.40 (ap s, 1 H), 4.34 (ap d, J = 10 Hz, 1 H), 4.08 (dd, J = 12.0, 3.6 Hz, 1 H), 3.99-3.94 (m, 1H), 3.96 (5, 3H), 3.67 (m, 1H), 2.52 (m, 2H), 2.06 (m, 1H), 1.93 (m,2H), 1 .77 (m, 2H), 1 .63 (m, 3H), 1 .50 (5, 3H), 1 .56 - 1 .42 (m, 4H), 1 .25 (m, 1 H),1.19 (t, J = 7.2 Hz, 3H), 1.09 (5, 9H), 1.10-0.93 (m, 2H), 0.85 (m, 2H), 0.69 (m,1 H), 0.49 (m, 1 H).
References:
WO2014/8285,2014,A1 Location in patent:Page/Page column 211; 212
1535212-03-3
2 suppliers
inquiry

1535212-07-7
43 suppliers
inquiry
![L-Valine,3-Methyl-N-[[[(1R,2R)-2-(4-penten-1-yl)cyclopropyl]oxy]carbonyl]-,Methyl ester](/CAS/20150408/GIF/1026200-25-8.gif)
1026200-25-8
1 suppliers
inquiry

1535212-07-7
43 suppliers
inquiry
![L-Valine, 3-Methyl-N-[[[(1R,2R)-2-(4-penten-1-yl)cyclopropyl]oxy]carbonyl]-](/CAS/20150408/GIF/1026200-27-0.gif)
1026200-27-0
6 suppliers
inquiry

1535212-07-7
43 suppliers
inquiry
![L-Valine, 3-methyl-N-[[[(1R,2R)-2-(4-oxobutyl)cyclopropyl]oxy]carbonyl]-](/CAS/20210305/GIF/1535210-59-3.gif)
1535210-59-3
0 suppliers
inquiry

1535212-07-7
43 suppliers
inquiry