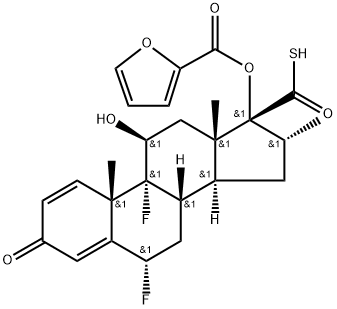
6α,9α-difluoro-17α-(furan-2-yl)carbonyloxy-11β-hydroxy-16α-methyl-3-oxoandrosta-1,4-diene-17β-carbothioic acid synthesis
- Product Name:6α,9α-difluoro-17α-(furan-2-yl)carbonyloxy-11β-hydroxy-16α-methyl-3-oxoandrosta-1,4-diene-17β-carbothioic acid
- CAS Number:397864-40-3
- Molecular formula:C26H28F2O6S
- Molecular Weight:506.56

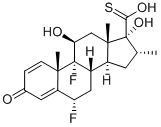
80473-92-3
79 suppliers
$198.00/50mg

397864-40-3
25 suppliers
inquiry
Yield:397864-40-3 102%
Reaction Conditions:
with triethylamine in N,N-dimethyl-formamide;
Steps:
Intermediate 3: 6α,9α-Difluoro-17α-[(2-furanylcarbonyl)oxy]-11β-hydroxy-16α-methyl-3-oxo-androsta-1,4-diene-17β-carbothioic Acid
A portion of the product (0.56 g) is mixed with 6α,9α-difluoro-11β, 17α-dihydroxy-16α-methyl-3-oxo-androsta-1,4-diene-17β-carbothioic acid (0.41 g) in a 1:1 molar ratio in DMF (10 volumes wrt total steroid input). The reaction mixture is treated with triethylamine (approximately 2.1 equivalents) and the mixture is stirred at approximately 20° C. for approximately 6 hours. Water (50 vol) containing excess conc HCl (0.5 vol) is added to the reaction mixture and the resultant precipitate collected by filtration. The bed is washed with water (2*5 vol) and dried in vacuo at approximately 55° C. overnight to leave the title compound as a white solid (0.99 g, 102%).
References:
US6777399,2004,B2
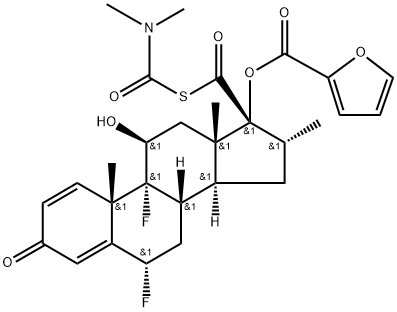
948565-92-2
25 suppliers
inquiry

397864-40-3
25 suppliers
inquiry
![Androsta-1,4-diene-17-carbothioic acid, 6,9-difluoro-17-[(2-furanylcarbonyl)oxy]-11-hydroxy-16-methyl-3-oxo-, anhydrosulfide with 2-furancarbothioic acid, (6α,11β,16α,17α)-](/CAS/20211123/GIF/397864-41-4.gif)
397864-41-4
2 suppliers
inquiry

397864-40-3
25 suppliers
inquiry

527-69-5
267 suppliers
$10.00/5g

80473-92-3
79 suppliers
$198.00/50mg

397864-40-3
25 suppliers
inquiry
![Androsta-1,4-diene-17-carbothioic acid, 6,9-difluoro-17-[(2-furanylcarbonyl)oxy]-11-hydroxy-16-methyl-3-oxo-, anhydrosulfide with 2-furancarbothioic acid, (6α,11β,16α,17α)-](/CAS/20211123/GIF/397864-41-4.gif)
397864-41-4
2 suppliers
inquiry
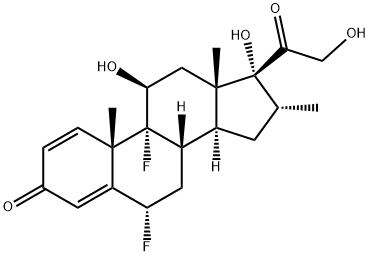
2135-17-3
386 suppliers
$31.00/50mg

397864-40-3
25 suppliers
inquiry