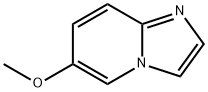
IMidazo[1,2-a]pyridine,6-Methoxy- synthesis
- Product Name:IMidazo[1,2-a]pyridine,6-Methoxy-
- CAS Number:955376-51-9
- Molecular formula:C8H8N2O
- Molecular Weight:148.16

107-20-0
4 suppliers
$22.60/5ml
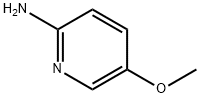
10167-97-2
219 suppliers
$18.00/1g
![IMidazo[1,2-a]pyridine,6-Methoxy-](/CAS/GIF/955376-51-9.gif)
955376-51-9
42 suppliers
$55.00/100mg
Yield:955376-51-9 90%
Reaction Conditions:
with Sodium hydrogenocarbonate at 25 - 80; for 6 h;
Steps:
72.1 Step 1: 6-methoxyimidazo[1,2-a]pyridine
To a solution of 5-methoxypyridin-2-amine (5 g, 40.28 mmol) in EtOH (30 mL) was added NaHCCb (6.77 g, 80.55 mmol, 3.13 mL) and 2-chloroacetaldehyde (15.81 g, 80.55 mmol, 0.25 mL, 40% purity) at 25 °C. The mixture was stirred at reflux (80 °C) for 6 hrs. The reaction mixture was concentrated in vacuum. The residue was dissolved with water (30 mL), washed with EtOAc (50 mL c 2), then basified with saturated Na2CC>3 to pH = 11. The aqueous solution was extracted with EtOAc (50 mL c 3). The combined organic phase was dried over Na2S04 and concentrated in vacuum. The residue was purified by column chromatography (SiCh, Petroleum ether: EtOAc=15:l to 10:1) to afford the title compound (5.4 g, 90% yield) as a brown oil. [0758] NMR (400 MHz, CD3OD) d = 7.97 - 7.93 (m, 1H), 7.63 (s, 1H), 7.37 (s, 1H), 7.30 (d, J = 10.0 Hz, 1H), 6.98 - 6.88 (m, 1H), 3.70 (d, J = 1.8 Hz, 3H).
References:
WO2021/154842,2021,A1 Location in patent:Paragraph 0755-0758

67-56-1
738 suppliers
$9.00/25ml
![6-IODOIMIDAZO[1,2-A]PYRIDINE](/CAS/GIF/426825-75-4.gif)
426825-75-4
88 suppliers
$28.00/250mg
![IMidazo[1,2-a]pyridine,6-Methoxy-](/CAS/GIF/955376-51-9.gif)
955376-51-9
42 suppliers
$55.00/100mg
6602-32-0
408 suppliers
$10.00/1g
![IMidazo[1,2-a]pyridine,6-Methoxy-](/CAS/GIF/955376-51-9.gif)
955376-51-9
42 suppliers
$55.00/100mg
24100-18-3
246 suppliers
$6.00/1g
![IMidazo[1,2-a]pyridine,6-Methoxy-](/CAS/GIF/955376-51-9.gif)
955376-51-9
42 suppliers
$55.00/100mg
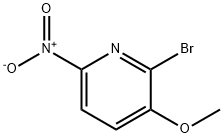
76066-07-4
92 suppliers
$44.00/250mg
![IMidazo[1,2-a]pyridine,6-Methoxy-](/CAS/GIF/955376-51-9.gif)
955376-51-9
42 suppliers
$55.00/100mg