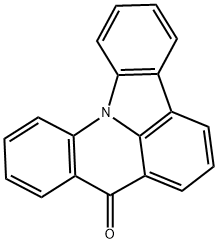
Indolo[3,2,1-de]acridin-8-one synthesis
- Product Name:Indolo[3,2,1-de]acridin-8-one
- CAS Number:32081-26-8
- Molecular formula:C19H11NO
- Molecular Weight:269.3
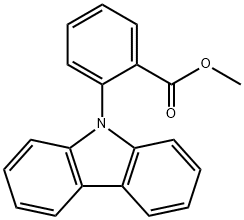
66131-59-7
2 suppliers
inquiry
![Indolo[3,2,1-de]acridin-8-one](/CAS/20180529/GIF/32081-26-8.gif)
32081-26-8
12 suppliers
inquiry
Yield:32081-26-8 60%
Reaction Conditions:
with polyphosphoric acid at 180; for 4 h;
Steps:
2.2.2 8H-indolo[3,2,1-de]acridin-8-one (3)
In a one-neck flask, carbazole (3.3g, 19.9mmol), methyl 2-iodobenzoate (5.7g, 21.8mmol), Cu (0.5g, 7.1mmol), CuI (0.34g, 1.8mmol) and K2CO3 (4.2g, 30.4mmol) were dissolved in 30mL 1,2-dichlorobenzene, then heated the mixture to reflux for 12h under nitrogen. After the reaction was completed fully, the mixture was allowed to cool to room temperature and then CH2Cl2 (50mL) was poured into the mixture. Filtered the mixture and washed the cake with CH2Cl2, concentrated the filtrate by a rotary evaporator, then recrystallization the product from the concentrated solution by ethanol to obtain a pale yellow solid 5.4g, 89%. 1H NMR (400MHz, CDCl3, TMS) δ: 8.12 (t, J=8.0Hz, 3H), 7.75 (t, J=7.6Hz, 1H), 7.59 (t, J=7.6Hz, 2H), 7.37 (t, J=7.6Hz, 2H), 7.26 (t, J=6.0Hz, 2H), 7.12 (d, J=8.2Hz, 2H), 3.18 (s, 3H). Moreover, in a one-neck flask, put the previous product methyl 2-(9H-carbazol-9-yl)benzoate (2.0g, 6.6mmol) into polyphosphoric acid (10mL), heated the mixture to 180°C for 4h. Cooled the mixture to about 80°C after the reaction had full completed, poured H2O (15mL) into the mixture and extracted with CH2Cl2 (20mL×3). The organic layer was dried over anhydrous MgSO4 and concentrated this using a rotary evaporator. The crude materials were purified by silica gel column chromatography (petroleum ether/CH2Cl2=3: 1 v/v) to obtain a pale yellow solid 1.2g, 60%. 1H NMR (400MHz, CDCl3, TMS) δ: 8.68 (dd, J=8.0, 1.6Hz, 1H), 8.45 (dd, J=7.6, 1.2Hz, 2H), 8.36 (dd, J=7.6, 1.2Hz, 1H), 8.30 (d, J=8.4Hz, 1H), 8.20-8.17 (m, 1H), 7.90-7.84 (m, 1H), 7.67-7.60 (m, 2H), 7.50-7.44 (m, 2H). 13C NMR (126MHz, CDCl3) δ: 134.10, 129.18, 127.92, 125.24, 124.52, 123.46, 123.21, 123.12, 121.64, 115.66, 114.11. HRMS (m/z): [M+H]- calcd for C19H11NO, 270.0919; found, 270.0915.
References:
Tian, Guojian;Liang, Wenqing;Chen, Yi;Xiang, Ning;Dong, Qingchen;Huang, Jinhai;Su, Jian-Hua [Dyes and Pigments,2016,vol. 126,p. 296 - 302]
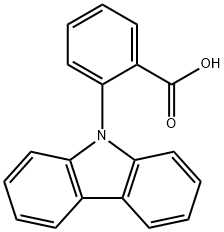
6286-88-0
4 suppliers
inquiry
![Indolo[3,2,1-de]acridin-8-one](/CAS/20180529/GIF/32081-26-8.gif)
32081-26-8
12 suppliers
inquiry
1150-62-5
295 suppliers
$8.00/5g
![Indolo[3,2,1-de]acridin-8-one](/CAS/20180529/GIF/32081-26-8.gif)
32081-26-8
12 suppliers
inquiry
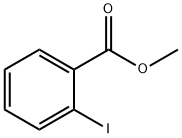
610-97-9
431 suppliers
$9.00/1g
![Indolo[3,2,1-de]acridin-8-one](/CAS/20180529/GIF/32081-26-8.gif)
32081-26-8
12 suppliers
inquiry
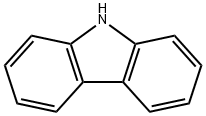
86-74-8
347 suppliers
$10.00/5g
![Indolo[3,2,1-de]acridin-8-one](/CAS/20180529/GIF/32081-26-8.gif)
32081-26-8
12 suppliers
inquiry