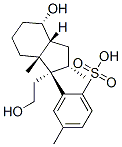
Inhoffen Lythgoe Diol Monotosylate synthesis
- Product Name:Inhoffen Lythgoe Diol Monotosylate
- CAS Number:66774-80-9
- Molecular formula:C20H30O4S
- Molecular Weight:366.51

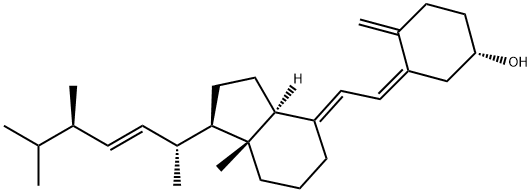
64190-52-9
95 suppliers
$180.00/50mg

98-59-9
585 suppliers
$9.00/5g

66774-80-9
24 suppliers
$180.00/250mg
Yield:66774-80-9 100%
Reaction Conditions:
with dmap;triethylamine in dichloromethane at 0; for 22 h;
Steps:
Preparation of De-A,B-20-methyl-pregnan-8β-ol (2) To a stirred solution of the diol 1 (1 g, 4.7 mmol), DMAP (50 mg, 0.4 mmol) and Et3N (1.96 mL, 1.42 g, 14.1 mmol) in anhydrous methylene chloride (25 mL), was added p-toluenesulfonyl chloride (1.07 g, 5.6 mmol) at 0° C. The reaction mixture was stirred at 0° C. for 22 hours. Methylene chloride (60 mL) was added, and the mixture was washed with water, dried (Na2SO4), and concentrated under reduced pressure. The residue was chromatographed on silica gel with hexane/ethyl acetate (9:1, then 85:15) to afford a tosylate (1.72 g, 100% yield) as a colorless oil.
References:
US2007/66566,2007,A1 Location in patent:Page/Page column 4-5
50-14-6
646 suppliers
$28.00/1g

66774-80-9
24 suppliers
$180.00/250mg

64190-52-9
95 suppliers
$180.00/50mg
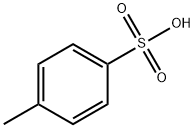
104-15-4
723 suppliers
$133.00/500g

66774-80-9
24 suppliers
$180.00/250mg