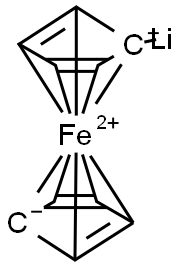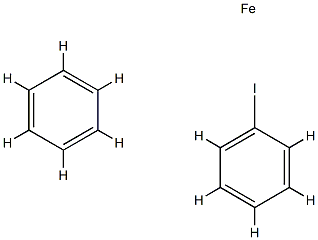
Iodoferrocene synthesis
- Product Name:Iodoferrocene
- CAS Number:1273-76-3
- Molecular formula:C12H11FeI
- Molecular Weight:337.9652
Yield:-
Reaction Conditions:
Stage #1:ferrocene with n-butyllithium;N,N,N,N,-tetramethylethylenediamine in hexane at 0 - 25; for 12 h;Inert atmosphere;
Stage #2: with iodine in hexane at -78 - 25; for 1 h;
Steps:
1
Step (1): Under a nitrogen system,10 g (53.8 mmol) of ferrocene was first dissolved in 50 mL of anhydrous n-hexane,Further, 18.1 mL (84.5 mmol) of tetramethylethylenediamine (TMEDA) was added,And 48.0 mL of n-hexane solution of 2.5 M n-butyllithium (n-BuLi) was slowly added dropwise at 0 ° C,And stirred at 25 ° C. After stirring for 12 hours,Remove the solvent first,And the resulting pale orange yellow complex was added to 200 mL of ethyl ether and the mixture was stirred and cooled to -78 ° C,Slowly drop the iodine ether solution (19.0 g I2 / 350 mL)Ether)After slowly warming to 25 ° C and stirring for 1 hour,The reaction was poured into 100 mL,5 wt% aqueous solution of ferric chloride (FeCl3)And then extracted with 200 mL of ether,The resulting organic layer was washed 10 times with 5 wt% of ferric chloride (FeCl3) aqueous solution (100 mL)And then washing the organic layer with water to the water layer is no longer discolored,To remove water with anhydrous magnesium sulfate (MgSO4) and remove the solvent,To obtain a mixture of compound a and compound b in a dark brown and liquid form(The molar ratio of compounds a and b is 1: 1; compounds a and b are shown in reaction I).
References:
Zheng, Jianhong;Lai, Zhenchang;Zhang, Yuwei;Liao, Chunyi;Huang, Minjie CN106317129, 2017, A Location in patent:Paragraph 0035; 0037
102-54-5
453 suppliers
$5.00/25g

1273-76-3
23 suppliers
inquiry

102-54-5
453 suppliers
$5.00/25g
7553-56-2
619 suppliers
$18.00/25g
12145-93-6
14 suppliers
inquiry

1273-76-3
23 suppliers
inquiry