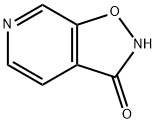
Isoxazolo[5,4-c]pyridin-3(2H)-one synthesis
- Product Name:Isoxazolo[5,4-c]pyridin-3(2H)-one
- CAS Number:847996-42-3
- Molecular formula:C6H4N2O2
- Molecular Weight:136.11
89640-77-7
23 suppliers
$435.00/500mg
![Isoxazolo[5,4-c]pyridin-3(2H)-one](/CAS2/GIF/847996-42-3.gif)
847996-42-3
11 suppliers
$634.27/1g
Yield:847996-42-3 96%
Reaction Conditions:
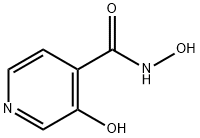
Stage #1: 3,N-dihydroxy-isonicotinamidewith 1,1'-carbonyldiimidazole in DMF (N,N-dimethyl-formamide) at 20;
Stage #2: with hydrogenchloride;water pH=4.5;Product distribution / selectivity;
Steps:
A.A
ISOXAZOLO [5, 4-CJPYRIDIN-3-OL (HIP) Method A; To a stirred suspension of 3, N-DIHYDROXY-ISONICOTINAMIDE (50 g; 0.32 mol) in DMF (300 mL), cooled on a water bath at ambient temperature, was added carbonyldiimidazole (DCI) (66.4 g; 0.41 mol. The reaction was stirred over night at ambient temperature. The next day the solvent was removed under reduced pressure at 70 °C and the residue was dissolved in water and cooled on ice. The pH of the solution (-7. 5) was adjusted to 4.5 by the addition of an aqueous solution of hydrochloric acid (ca. 65 mL, 10 M). The desired product crystallised heavily from the solution. The slurry was evaporated to ca. 2/3 volume to remove traces of DMF, cooled on ice for a couple of hours and filtered. The crystals so obtained were rinsed twice with water and twice with ethanol and dried under reduced pressure at 60 °C overnight to give isoxazolo [5,4-c] pyridin-3-ol (41.7 g, 96 %; HPLC purity >99 %) as a white solid. NMR data:'H-NMR (DMSO-d6,250 MHz) 8 = 7.84 (1H, d, J=6 Hz), 8.53 (1H, d, J=6 Hz), 9.08 (1H, s) ppm.
References:
WO2005/23820,2005,A1 Location in patent:Page/Page column 16-17