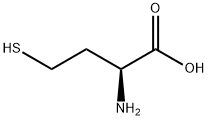
L-HOMOCYSTEINE synthesis
- Product Name:L-HOMOCYSTEINE
- CAS Number:6027-13-0
- Molecular formula:C4H9NO2S
- Molecular Weight:135.18
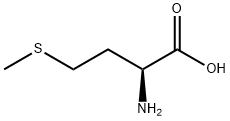
63-68-3
898 suppliers
$5.00/25g

6027-13-0
142 suppliers
$22.00/1mg
Yield:-
Reaction Conditions:
Stage #1:L-methionine with ammonia;sodium at -78;Inert atmosphere;
Stage #2: with ammonium acetate in ammoniaInert atmosphere;
Steps:
11.1
Synthetic Example 11; (S)-2-(Pyrimidin-2-ylamino)-4-mercapto-butyric acid, sodium salt; Step 1. (S)-2-Amino-4-mercapto-butyric acid; A 1.5-liter round bottom flask, equipped with a mechanical stirrer, was charged with L-methionine (20 g, 134 mmol). The reaction flask was flushed with argon and cooled to -780C with a dry ice-acetone bath. Anhydrous ammonia gas was condensed in the flask until the starting material was completely dissolved. The dry ice bath was removed, and sodium metal (11 g, 483 mmol) was added in pieces to the refluxing ammonia solution. The progress of the reaction was monitored by quenching a small aliquot of the reaction and analyzing via TLC and HPLC. After the reaction was complete, any visible remaining pieces of sodium metal were carefully removed and ammonium acetate (9.46 g, 130 mmol) was added in portions to quench the reaction mixture. The ammonia was evaporated overnight under a stream of argon. The resulting solid of (S)-2-amino-4-mercapto-butyric acid was ground with a mortar and pestle, and used in the next step without purification.
References:
GALLEON PHARMACEUTICALS, INC. WO2009/151744, 2009, A1 Location in patent:Page/Page column 102
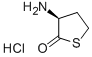
31828-68-9
86 suppliers
$89.59/100mg

6027-13-0
142 suppliers
$22.00/1mg
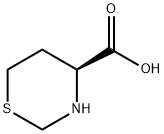
147331-83-7
4 suppliers
inquiry

6027-13-0
142 suppliers
$22.00/1mg
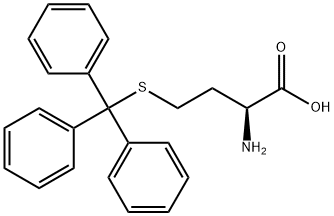
69955-57-3
2 suppliers
inquiry

6027-13-0
142 suppliers
$22.00/1mg

7689-60-3
17 suppliers
$75.00/25 mg

6027-13-0
142 suppliers
$22.00/1mg