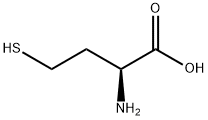
DL-Homocysteine synthesis
- Product Name:DL-Homocysteine
- CAS Number:454-29-5
- Molecular formula:C4H9NO2S
- Molecular Weight:135.18

1192-20-7
23 suppliers
$45.00/10mg

454-29-5
224 suppliers
$25.00/10mg
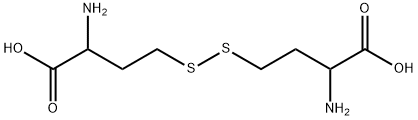
462-10-2
95 suppliers
$9.00/5g
Yield:454-29-5 36 %Chromat. ,462-10-2 14 %Chromat.
Reaction Conditions:
with sodium sulfide;carbon dioxide in 1-methyl-pyrrolidin-2-one at 20 - 90; for 0.666667 h;
Steps:
3 EXAMPLE 3 Preparation of Homocysteine According to the Invention
EXAMPLE 3 Preparation of Homocysteine According to the Invention With the method of the invention, it is also possible to obtain the dimer of homocysteine (called homocystine). [0033] This example is carried out on a scale of 1 mmol of 2ABL in a pill box with magnetic stirring. The 2-ABL is placed in solution in 1.2 mL of NMP. CO2 bubbling is carried out at 20° C. for 10 mins, and then 10.8 mL of NMP and 3 equivalents of Na2S are added. The reaction medium placed under stirring is gradually heated up to 90° C. After 30 mins, the medium is hydrolyzed by simple dilution in the HPLC solvent. [0034] These conditions allow formation of homocysteine and of its dimer. The best performances are obtained at a temperature of 90° C. [0035] After a reaction time of 30 mins, the reaction is completed. At a higher temperature and in the presence of 3 equivalents of Na2S, homocystine is favored. Excess Na2S accelerates dimerization of homocysteine. [0036] The following yields (HPLC) are obtained: Homocysteine 36% Homocystine 14% Diketopiperazine 2%
References:
ADISSEO FRANCE S.A.S.;Huet, Robert;Joerger, Jean-Michel;Henryon, Vivien US2013/184474, 2013, A1 Location in patent:Paragraph 0031; 0032; 0033; 0034; 0035; 0036
59-51-8
800 suppliers
$5.00/100mg

454-29-5
224 suppliers
$25.00/10mg
6038-19-3
317 suppliers
$6.00/25g

454-29-5
224 suppliers
$25.00/10mg

462-10-2
95 suppliers
$9.00/5g

454-29-5
224 suppliers
$25.00/10mg

1017-76-1
16 suppliers
inquiry

454-29-5
224 suppliers
$25.00/10mg