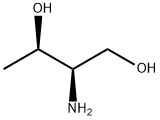
L-Threoninol synthesis
- Product Name:L-Threoninol
- CAS Number:3228-51-1
- Molecular formula:C4H11NO2
- Molecular Weight:105.14
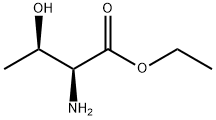
23926-51-4
4 suppliers
inquiry

3228-51-1
145 suppliers
$40.00/1g
Yield:3228-51-1 97%
Reaction Conditions:
with lithium aluminium hydride in tetrahydrofuran; for 3 h;Reflux;
Steps:
1.2.2 (R,R)-Threoninol
Lithium aluminum hydride (LAH, 3.56g, 94mmol) was suspended in 200mL of THF. L- threonine ethyl ester (3.45g, 23mmol) was dissolved in 50mL of THF and added dropwise to the suspension of LAH/THF over 1.5h. The mixture was refluxed for 3h. Afterward, the solution was ice-cooled and the remaining LAH was quenched with 0.1N HCl. The lithium salts were then removed by filtration. The filtrate was concentrated by vacuum rotary evaporation to give (R,R)-threoninol (2.40g, 22.6mmol) in 97% yield. (0008) TLC: Rf 0.10 (60:40, methanol/ethyl acetate (v/v) on silica). 1H NMR (300MHz, CDCl3, in ppm relative to TMS): δ 3.51 (dq, J=6.9, 5.9Hz, 1H, H2); [3.35 (AB of ABX, J=6.4, 5.2 Hz, 1H, H4); 3.20 (AB of ABX, J=6.4, 5.2Hz, 1H, H4’)]; 2.36 (m, 1H, H3); 1.00 (d, J=6.9Hz, 3H, H1). 13C NMR (75MHz, CDCl3, in ppm relative to TMS) δ (66.7, C2); (63.6, C4); (58.3, C3); (20.3, C1). HR-FAB MS, C4H11NO2 ([M+H]+): expected 105.0790, found 105.0801.
References:
Putnam, William C.;Bashkin, James K. [Journal of Inorganic Biochemistry,2022,vol. 232,art. no. 111831]
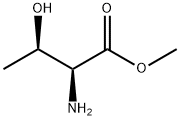
3373-59-9
58 suppliers
$44.00/5g

3228-51-1
145 suppliers
$40.00/1g
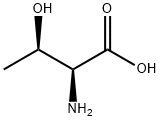
72-19-5
681 suppliers
$4.76/25g

3228-51-1
145 suppliers
$40.00/1g
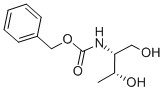
161150-55-6
1 suppliers
inquiry

3228-51-1
145 suppliers
$40.00/1g