
Leniolisib synthesis
- Product Name:Leniolisib
- CAS Number:1354690-24-6
- Molecular formula:C21H25F3N6O2
- Molecular Weight:450.46
![1-Pyrrolidinecarboxylic acid, 3-[[5,6,7,8-tetrahydro-6-[6-methoxy-5-(trifluoromethyl)-3-pyridinyl]pyrido[4,3-d]pyrimidin-4-yl]amino]-, 1,1-dimethylethyl ester, (3S)-](/CAS/20210111/GIF/1354691-73-8.gif)
1354691-73-8
1 suppliers
inquiry
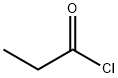
79-03-8
411 suppliers
$9.00/1g

1354690-24-6
77 suppliers
$39.00/1mg
Yield: 76%
Reaction Conditions:
Stage #1:(S)-3-[6-(6-methoxy-5-trifluoromethyl-pyridin-3-yl)-5,6,7,8-tetrahydro-pyrido[4,3-d]pyrimidin-4-ylamino]-pyrrolidine-1-carboxylic acid tert-butyl ester with trifluoroacetic acid in dichloromethane at 20; for 1 h;
Stage #2:propionyl chloride in dichloromethane at 20; for 1.33 h;Concentration;Reagent/catalyst;Temperature;
Steps:
67
Example 67: 1-{(S)-3-[6-(6-Methoxy-5-trifluoromethyl-pyridin-3-yl)-5,6,7,8-tetrahydro- pyrido[4,3-d]pyrimidin-4-ylamino]-pyrrolidin-1 -yl}-propan-1 -one To a solution of (S)-3-[6-(6-methoxy-5-trifluoromethyl-pyridin-3-yl)-5,6,7,8-tetrahydro- pyrido[4,3-d]pyrimidin-4-ylamino]-pyrrolidine-1-carboxylic acid tert-butyl ester (intermediate 24) (13.4 g, 27.1 mmol) in CH2CI2 (100 mL), was added TFA (41.8 mL) and the mixture stirred at rt for 1 h. Concentrated in vacuo and partitioned between 2M NaOH(aq) (300 mL) and CH2CI2 (200 mL). The organic phase was separated and the aqueous phase extracted with CH2CI2 (2 x 200 mL). The organic phases were combined, dried (MgS04) andevaporated in vacuo to give a brown foam. The foam was dissolved in CH2CI2 (50 mL) and was added simultaneously portionwise with sat.NaHC03(aq) (50 mL) to a vigourously stirring solution of propionyl chloride (2.63 g, 28.5 mmol) in CH2CI2 (50 mL) at rt. The resulting biphasic mixture was stirred at rt for 1 h. Further propionyl chloride (0.566g, 6.12 mmol) was added and continued stirring vigorously for 20 min. The organic layer was separated and the aqueous layer extracted with CH2CI2 (100 mL). The organic layers were combined, dried (MgS04) and concentrated in vacuo to give a brown gum. The gum was stirred in EtOAc (100 mL) and the resulting solid filtered (9.4 g). The mother liquors were concentrated in vacuo and purified by column chromatography through a Biotage amino silica gel eluting with EtOAc / MeOH, 100/0 to 90/10 to give a yellow foam which was then stirred in EtOAc (20 mL) and the resulting solid filtered (870 mg). Both batches of solids were combined and stirred in refluxing EtOAc (50 mL) for 1 h. Filtered to give 1 -{(S)-3-[6-(6-methoxy-5- trifluoromethyl-pyridin-3-yl)-5,6,7,8-tetrahydro-pyrido[4,3-d]pyrimidin-4-ylamino]-pyrrolidin-1- yl}-propan-1 -one as a colourless solid (9.42 g, 76% yield). 1H NMR (400 MHz, DMSO-d6, 298K) δ ppm 0.95-1.05 (m, 3H) 1.87-2.32 (m, 4H) 2.77-2.86 (m, 2H) 3.25-3.88 (m, 6H) 3.93 (s, 3H) 3.98 (s, 2H) 4.55-4.80 (m, 1 H) 6.70-6.80 (m, 1 H, N-H) 7.86-7.92 (m, 1 H) 8.27-8.33 (m, 1 H) 8.33-8.37 (m, 1 H) LCMS: [M+H]+=451 .0, Rt (6)= 1 .49 min.
References:
NOVARTIS AG;FERNANDES GOMES DOS SANTOS, Paulo Antonio;HÖGENAUER, Klemens;HOLLINGWORTH, Gregory;SOLDERMANN, Nicolas;STOWASSER, Frank;TUFILLI, Nicola;ZECRI, Frédéric WO2013/1445, 2013, A1 Location in patent:Page/Page column 58-59
![6-Benzyl-4-chloro-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine](/CAS/GIF/914612-23-0.gif)
914612-23-0
108 suppliers
$24.00/100mg

1354690-24-6
77 suppliers
$39.00/1mg
![1-Pyrrolidinecarboxylic acid, 3-[[5,6,7,8-tetrahydro-6-(phenylmethyl)pyrido[4,3-d]pyrimidin-4-yl]amino]-, 1,1-dimethylethyl ester, (3S)-](/CAS/20210111/GIF/1354691-71-6.gif)
1354691-71-6
1 suppliers
inquiry

1354690-24-6
77 suppliers
$39.00/1mg
![1-Pyrrolidinecarboxylic acid, 3-[(5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-4-yl)amino]-, 1,1-dimethylethyl ester, (3S)-](/CAS/20210111/GIF/1354691-72-7.gif)
1354691-72-7
1 suppliers
inquiry

1354690-24-6
77 suppliers
$39.00/1mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

79-03-8
411 suppliers
$9.00/1g

1354690-24-6
77 suppliers
$39.00/1mg