Lercanidipine Impurity 26 synthesis
- Product Name:Lercanidipine Impurity 26
- CAS Number:890045-70-2
- Molecular formula:C20H23ClN2O6
- Molecular Weight:422.86
Yield:890045-70-2 58%
Reaction Conditions:
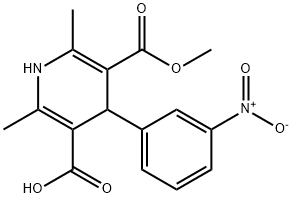
Stage #1: 5-methoxycarbonyl-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3-carboxylic acidwith thionyl chloride in dichloromethane;N,N-dimethyl-formamide at -2 - 2; for 1 h;
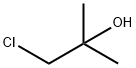
Stage #2: 3-chloro-2-methylpropan-2-ol in dichloromethane;N,N-dimethyl-formamide at -5 - 20; for 27 h;
Steps:
1
Example 1; l-Chloro-2-methyl-2-propyl methyl 1 ,4-dihydro-2,6-dimethyl-4-(3- nitrophenyl)- 1 -pyridine-3 , 5 -dicarboxylate; 31 g (0.26 moles) of thionyl chloride were added dropwise under stirring to a mixture of 78 g (0.235 moles) of 2,6-dimethyl-5-methoxycarbonyl-4- (3-nitrophenyl)-l,4-dihydropyridine-3-carboxylic acid, 420 ml methylene dichloride and 110 ml dimethyl formamide kept at temperature of -20C - +2° under nitrogen. After terminating the addition of the thionyl chloride the stirring was continued under nitrogen for a further hour. To the above mixture a solution of 26 g (0.24 moles) of l-chloro-2-methyl-2-propanol in 60 ml methylene dichloride was added dropwise while stirring under nitrogen at a temperature of -50C - O0C. The stirring was continued for 3 EPO
References:
WO2006/59332,2006,A1 Location in patent:Example 1