
m-PEG3-Tos synthesis
- Product Name:m-PEG3-Tos
- CAS Number:50586-80-6
- Molecular formula:C12H18O5S
- Molecular Weight:274.33
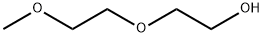
111-77-3
448 suppliers
$10.60/50gm:

98-59-9
585 suppliers
$9.00/5g

50586-80-6
65 suppliers
$79.00/1g
Yield: 100%
Reaction Conditions:
with pyridine at 0 - 20; for 2 h;
Steps:
9.1
To a solution of diethylene glycol monomethylether(12 g, 100 mmol) in pridine(70 ml) was added p-toluenesulfonyl chloride(22.8 g, 120 mmol) at 0° C., which was then stirred at room temperature for 2 hours. Water was added thereto, and the mixture was extracted with diethyl ether. The extracted organic layer was washed with 0.5N-HCl, water and saturated sodium bicarbonate solution, and dried over anhydrous magnesium sulfate. The organic solvent was removed under reduced pressure to give the title compound (27 g, stoichiometric yield) as a yellow oil. The compound thus obtained was used in the next reaction without further purification. [00564] 1H-NMR (270 MHz, CDCl3) δ: 3.54 (m, 6H, 3×OCH2), 3.35 (s, 3H, OCH3), 3.07 (t, J=6.2 Hz, 2H, SCH2), 2.31 (s, 3H, SCOCH3)
References:
Chugai Seiyaku Kabushiki Kaisha US6645951, 2003, B1 Location in patent:Page/Page column 83
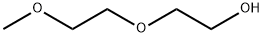
111-77-3
448 suppliers
$10.60/50gm:
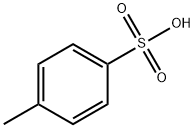
104-15-4
723 suppliers
$133.00/500g

50586-80-6
65 suppliers
$79.00/1g

