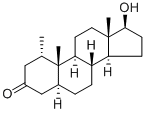
Mesterolone synthesis
- Product Name:Mesterolone
- CAS Number:1424-00-6
- Molecular formula:C20H32O2
- Molecular Weight:304.47
Mesterolone is a synthetic androgen and a dihydrotestosterone derivative. Mesterolone is rarely used for replacement therapies due to its weak androgenic activity. It can be synthesized by mixing 1α-methyl-androstan-17β-ol-3-one-17-acetate with methanolic sodium hydroxide.
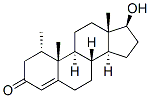
604-26-2
5 suppliers
$151.00/y0000096

1424-00-6
294 suppliers
$39.00/1mg
Yield:1424-00-6 98%
Reaction Conditions:
Stage #1: 17β-hydroxy-1α-methyl-androst-4-en-3-onewith ammonia;lithium in tetrahydrofuran;tert-butyl alcohol at -50 - -45;Birch Reduction;
Stage #2: with 4-methyl-pent-3-en-2-one in tetrahydrofuran;tert-butyl alcohol;
Steps:
1 Preparation of mesterolone (17β-hydroxy-1α-methylandrostan-3-one)
EXAMPLE 1 Preparation of mesterolone (17β-hydroxy-1α-methylandrostan-3-one) In a mixture of 50 ml of THF, 19.0 ml of t-butanol and 40 ml of ammonia, 2.01 g (289.58 mmol) of lithium are dissolved at -45° C. The mixture is admixed at -50° C. with a solution of 32 g (105.79 mmol) of 1α-methyl testosterone in 210 ml of THF. After the reaction has ended, it is quenched by adding 10 ml of mesityl oxide. The reaction mixture is warmed to +20° C. and added to a suspension of 16 g of ammonium chloride and 50 ml of water. After phase separation, the organic phase is introduced into 2 l of ice-water. The resulting suspension is adjusted to pH=approx. 3 with HCl. Thereafter, the precipitated solid is isolated, washed to neutrality with water and dried at 50° C. Yield: 98-100% of weight of starting material.
References:
US2008/300428,2008,A1 Location in patent:Page/Page column 1
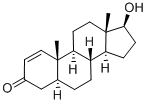
65-06-5
99 suppliers
$136.00/500μg
676-58-4
282 suppliers
$15.00/25ml

1424-00-6
294 suppliers
$39.00/1mg
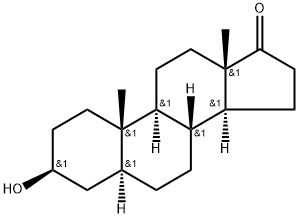
481-29-8
348 suppliers
$39.00/5mg

1424-00-6
294 suppliers
$39.00/1mg
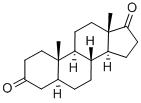
846-46-8
75 suppliers
inquiry

1424-00-6
294 suppliers
$39.00/1mg
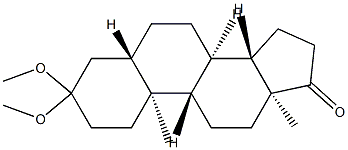
3591-19-3
6 suppliers
inquiry

1424-00-6
294 suppliers
$39.00/1mg