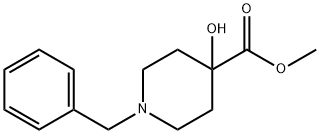
Methyl 1-benzyl-4-hydroxypiperidine-4-carboxylate synthesis
- Product Name:Methyl 1-benzyl-4-hydroxypiperidine-4-carboxylate
- CAS Number:60437-30-1
- Molecular formula:C14H19NO3
- Molecular Weight:249.31

67-56-1
738 suppliers
$7.29/5ml-f

6094-60-6
62 suppliers
$14.00/1g

60437-30-1
51 suppliers
$95.00/250mg
Yield: 90%
Reaction Conditions:
Stage #1:1-benzyl-4-hydroxypiperidine-4-carbonitrile with hydrogenchloride;water at 90; for 1 h;
Stage #2:methanol;sulfuric acid at 50; for 1 h;
Steps:
1A Methyl 1-benzyl-4-hydroxypiperidine-4-carboxylate
Example 1A Methyl 1-benzyl-4-hydroxypiperidine-4-carboxylate A solution of 10.52 g (48.64 mmol) of 1-benzyl-4-hydroxypiperidine-4-carbonitrile in 60 ml of conc. hydrochloric acid is stirred for one hour at 90° C. The reaction solution is concentrated on a rotary evaporator and dried under high vacuum. The residue obtained is taken up in 150 ml of methanol, 6 ml of conc. sulfuric acid are added and the mixture stirred for 1 hour at 50° C. After cooling the reaction mixture is diluted with ethyl acetate and rendered alkaline with a saturated sodium carbonate solution. The organic phase is washed with a sodium chloride solution, dried over sodium sulfate and concentrated on a rotary evaporator. 10.8 g (43.6 mmol, 90% th.) of product are obtained. LC-MS (method 4): Rt=2.08 min. MS (ESIpos): m/z=250 (M+H)+ 1H NMR (400 MHz, DMSO-d6): δ=7.35-7.2 (m, 5H), 5.28 (s, 1H), 3.63 (s, 3H), 3.45 (s, 2H), 2.53-2.4 (m, 2H, partly masked by DMSO), 2.38-2.2 (m, 2H), 1.9-1.78 (m, 2H), 1.59 (d, 2H).
References:
Location in patent:Page/Page column 16

6094-60-6
62 suppliers
$14.00/1g

60437-30-1
51 suppliers
$95.00/250mg

67-56-1
738 suppliers
$7.29/5ml-f
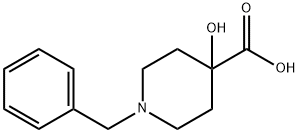
59119-18-5
50 suppliers
$45.00/25mg

60437-30-1
51 suppliers
$95.00/250mg
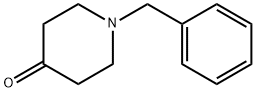
3612-20-2
490 suppliers
$6.00/10g

60437-30-1
51 suppliers
$95.00/250mg

67-56-1
738 suppliers
$7.29/5ml-f

3612-20-2
490 suppliers
$6.00/10g
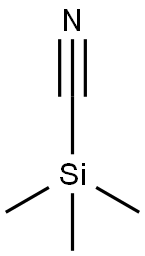
7677-24-9
374 suppliers
$19.00/5g

60437-30-1
51 suppliers
$95.00/250mg