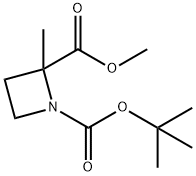
Methyl 1-Boc-2-Methylazetidine-2-carboxylate synthesis
- Product Name:Methyl 1-Boc-2-Methylazetidine-2-carboxylate
- CAS Number:309977-81-9
- Molecular formula:C11H19NO4
- Molecular Weight:229.27
255882-72-5
113 suppliers
$34.00/1g

74-88-4
343 suppliers
$15.00/10g

309977-81-9
34 suppliers
$155.00/100mg
Yield:309977-81-9 70%
Reaction Conditions:
with N,N,N,N,N,N-hexamethylphosphoric triamide;n-butyllithium;ammonium chloride;diisopropylamine in tetrahydrofuran;
Steps:
143 Step A.
To a solution of diisopropylamine (1.05 equiv; 5.9 mmol, 0.6 g, 0.83 mL) in anhydrous tetrahydrofuran (15 mL) was added n-butyl lithium (1.05 equiv; 5.9 mmol, 2.36 mL of a 2.5M solution in hexanes) and the resulting light yellow solution was warmed to 0° C. for 10 min. It was then cooled back to -78° C. and N-(tert-butoxycarbonyl)-azetidine-2(S)-carboxylic acid, methyl ester (5.63 mmol, 1.21 g) in dry tetrahydrofuran (3.0 mL) was added dropwise and stirring was continued for 45 min. After this time, hexamethylphosphoramide (2.0 equiv; 11.3 mmol, 2.02 g, 1.96 mL) was added and the reaction mixture was stirred for 0.5 h. At this point, iodomethane (5.0 equiv; 28.2 mmol, 3.99 g, 1.75 mL) was added (-78° C.), the cooling bath was removed, and stirring was continued overnight (16 h) at ambient temperature. Addition of saturated ammonium chloride solution, extraction with ethyl acetate, washing of the combined organic layer with water (2*) and brine, drying (MgSO4), and concentration yielded crude material which was purified by Biotage flash-coloumn chromatography (15% ethyl acetate, 85% hexanes) to provide N-(tert-butyloxycarbonyl)-2(R,S)-methylazetidine-2-carboxylic acid, methyl ester (0.9 g, 70% yield). 1H NMR (400 MHz, CDCl3): δ 4.95 (m, 1H), 3.80 (m, 1H), 3.78 (s, 3H), 2.35 (m, 1H), 2.18 (m, 1H), 1.69 (s, 3H; one rotamer), 1.63 (s, 3H; other rotamer), 1.46 (s, 9H; one rotamer), 1.42 (s, 9H; other rotamer)
References:
US6645939,2003,B1