
methyl 2-(4-chlorophenyl)-2-oxoacetate synthesis
- Product Name:methyl 2-(4-chlorophenyl)-2-oxoacetate
- CAS Number:37542-28-2
- Molecular formula:C9H7ClO3
- Molecular Weight:198.6

5781-53-3
219 suppliers
$8.00/10g
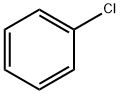
108-90-7
644 suppliers
$10.00/25G

37542-28-2
37 suppliers
$19.00/250mg
Yield:37542-28-2 99 g
Reaction Conditions:
Stage #1: monomethyl oxalyl chloridewith aluminum (III) chloride in chloroform at 0; for 1 h;Inert atmosphere;Friedel-Crafts acylation;
Stage #2: chlorobenzene in chloroform at 0 - 20;Inert atmosphere;Friedel-Crafts acylation;
Steps:
4.1.6. 4-Chloro-phenylglyoxylic acid (14)
Methyl oxalyl chloride (265 g, 2.16 mol) was slowly added to a suspension of aluminium chloride (200 g, 1.5 mol) in 750 ml of chloroform at 0 °C. The reaction mixture was stirred for 1 h at 0 °C, then chlorobenzene (245 g, 2.19 mol) was slowly added at this temperature. The resulting mixture was stirred for 16 h at room temperature, then poured on water and diluted with dichloromethane. The phases were separated, the organic layer was washed with water, dried over sodium sulfate and evaporated. The remainder was purified by chromatography on silica gel, using ethyl acetate/heptane 1:9 as eluent, delivering methyl 4-chloro-phenylglyoxylate (99 g, 0.5 mol). This intermediate was dissolved in 800 ml of dioxane and 800 ml of 1 N sodium hydroxide were added. The mixture was stirred for 2 h at room temperature, then acidified to pH 1 with 2 N hydrochloric acid and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and evaporated to deliver 4-chloro-phenylglyoxylic acid (14, 86 g, 0.47 mol 21%). 1H NMR (CDCl3): δ = 7.53 (d, 2H), 8.10 (br s, 1H), 8.32 (d, 2H). MS (ESI): m/z = 186 (M+1), 165 (M-OH).
References:
Lamberth, Clemens;Trah, Stephan;Wendeborn, Sebastian;Dumeunier, Raphael;Courbot, Mikael;Godwin, Jeremy;Schneiter, Peter [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 9,p. 2803 - 2810] Location in patent:experimental part
52449-43-1
186 suppliers
$6.00/5g

37542-28-2
37 suppliers
$19.00/250mg
93961-29-6
1 suppliers
inquiry

37542-28-2
37 suppliers
$19.00/250mg

67-56-1
738 suppliers
$9.00/25ml
4640-66-8
248 suppliers
$6.00/1g

37542-28-2
37 suppliers
$19.00/250mg
99-91-2
429 suppliers
$10.00/25g

74-88-4
342 suppliers
$15.00/10g

37542-28-2
37 suppliers
$19.00/250mg