
Methyl 2,6-dichloro-4-nitrobenzoate synthesis
- Product Name:Methyl 2,6-dichloro-4-nitrobenzoate
- CAS Number:232275-50-2
- Molecular formula:C8H5Cl2NO4
- Molecular Weight:250.04
Yield:232275-50-2 84%
Reaction Conditions:
Stage #1: 2,6-dichloro-4-nitrobenzoic acidwith thionyl chloride;N,N-dimethyl-formamide in 1,2-dichloro-ethane; for 2 h;Reflux;
Stage #2: methanol at 50; for 20 h;
Steps:
Preparation of methyl 2,6-dichloro-4-nitrobenzoate (5)
A mixture of 4 (1.24 g, 4.75 mmol) and DMF (one drop) in 1,2-dichloroethane (10 mL) was stirred at 25 °C for 5 min. Thionyl chloride (5.8 mL, 79.5 mmol) was added to the mixture, which was then refluxed for 2 h. After cooling, the mixture was concentrated in vacuo. Methanol (10 mL) was added to the residue and the mixture was stirred at 50 °C for 20 h. Brine (20 mL) was added to the mixture and the product was extracted with chloroform (3 × 20 mL). The combined organic layer was washed with brine (2 × 20 mL), dried over Na2SO4 (10 g) and concentrated in vacuo. The crude residue was purified by silica gel (20 g) column chromatography with hexane/chloroform = 2/1 as an eluent to give 5 (1.00 g, 84%) as a pale yellow solid.
References:
Nishio, Yuya;Kawazu, Akari;Hirano, Shun;Matsubara, Hiroshi [Tetrahedron,2016,vol. 72,# 5,p. 720 - 725] Location in patent:supporting information
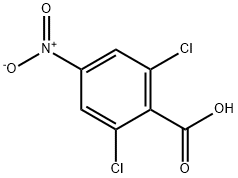
22509-50-8
22 suppliers
inquiry

232275-50-2
8 suppliers
inquiry
7149-69-1
97 suppliers
$19.00/250mg

232275-50-2
8 suppliers
inquiry
99-99-0
314 suppliers
$5.00/5G

232275-50-2
8 suppliers
inquiry

