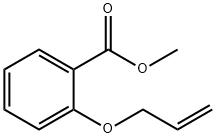
Methyl 2-(allyloxy)benzoate synthesis
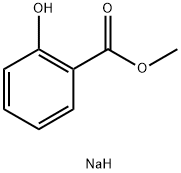
- Product Name:Methyl 2-(allyloxy)benzoate
- CAS Number:6282-42-4
- Molecular formula:C11H12O3
- Molecular Weight:192.21
Yield:6282-42-4 100%
Reaction Conditions:
with potassium carbonate in water;acetonitrile at 50; for 48 h;Reagent/catalyst;
Steps:
Methyl 2-(allyloxy)benzoate (12)
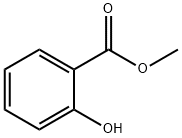
Methyl salicylate (4) (1.70 mL, 13.1 mmol, 1.0 eq.) and potassium carbonate(3.64 g, 26.2 mmol, 2.0 eq.) were dissolved in acetonitrile (50 mL). Allylbromide (2.30 mL, 26.2 mmol, 2.0 eq.) was then slowly added. The reactionmixture was stirred for two days at 50 °C. The excess salt was filtered offand the solvent removed under reduced pressure. The residue was taken up into diethyl ether andsaturated NaHCO3 solution. The aqueous phase was separated from the organic phase and extractedtwice with about 20 mL diethylether. The combined organic phases were dried over magnesiumsulfate, filtered and the solvent was removed under reduced pressure. Allyl phenyl ether 12 (2.56 g,13.3 mmol; quantitative) was obtained as a yellow oil.1H-NMR (400 MHz, CDCl3, CHCl3 = 7.26 ppm): [ppm] = 7.80 (1H, dd, J = 7.7 Hz, J = 1.9 Hz, H-9), 7.46-7.41 (1H, m, arom.), 7.00-6.95 (2H, m, arom.), 6.11-6.02 (1H, m, H-2), 5.51 (1H, dq, J =17.2 Hz, J = 1.8 Hz, H-1), 5.30 (1H, dq, J = 10.6 Hz, J = 1.6 Hz, H-1), 4.63 (2H, dt, J = 4.9 Hz, J =1.8 Hz, H-3), 3.90 (3H, s, H-13); 13C-NMR (100 MHz, CDCl3, CDCl3 = 77.16 ppm): [ppm] = 166.9 (q, C-11), 158.2 (q, C-5), 133.5 (t, arom.), 132.9 (t, C-2), 131.9 (t, C-9), 120.7 (q, C-10), 120.6 (t,arom.), 117.5 (s, C-1), 113.8 (t, arom.), 69.6 (s, C-3), 52.1 (p, C-13); HRMS (EI): m/z calculated forC11H12O3: 192.0786; found 192.0787; Rf (9:1 pentane/Et2O): 0.20.
References:
Oltmanns, Mona;Kirschning, Andreas [Synlett,2020,vol. 31,# 19,p. 1942 - 1946] Location in patent:supporting information
69-72-7
1261 suppliers
$5.00/10g

6282-42-4
6 suppliers
inquiry

67-56-1
757 suppliers
$9.00/25ml

59086-52-1
27 suppliers
$175.00/500mg

6282-42-4
6 suppliers
inquiry