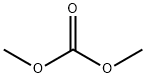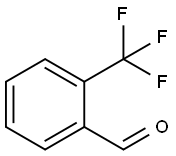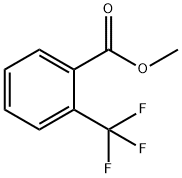
Methyl 2-(trifluoromethyl)benzoate synthesis
- Product Name:Methyl 2-(trifluoromethyl)benzoate
- CAS Number:344-96-7
- Molecular formula:C9H7F3O2
- Molecular Weight:204.15
Yield: 80.7 %Chromat.
Reaction Conditions:
Stage #1:1-chloro-2-(trifluoromethyl)benzene with ethyl bromide;magnesium;lithium chloride in tetrahydrofuran at 45 - 50; for 5 h;Inert atmosphere;
Stage #2:carbonic acid dimethyl ester in tetrahydrofuran;toluene at 40 - 50; for 7 h;Inert atmosphere;
Stage #3: with hydrogenchloride in tetrahydrofuran;water;toluene at 20; for 1 h;Inert atmosphere;
Steps:
1 Example 1
Tetrahydrofuran 75.0 g (1.04 mol; manufactured by nacalai tesque), Magnesium powder 5.1 g (0.208 mol; manufactured by Chuo Kogyo), 1.7 g of LiCl (0.04 mol; manufactured by nacalai tesque) Was put into 200 ml of a four-necked flask equipped with a thermometer, and while replacing the inside of the system with nitrogen, Followed by stirring. To this was added 1 mol / L ethylmagnesium bromide THF solution 0.5 g (Tokyo Chemical Industry Co., Ltd.) was added to remove moisture in the system. continue, 0.44 g (0.004 mol; manufactured by Wako Pure Chemical Industries, Ltd.) of ethyl bromide was added. It was stirred for a while and it was confirmed that heat generation occurred. While maintaining the temperature of the reaction solution at 45 to 50 ° C., O-chlorobenzotrifluoride 36.1 g (0.2 mol; manufactured by Wako Pure Chemical Industries, Ltd.) Was slowly added dropwise. After completion of the dropwise addition, aging was carried out while stirring at 45 ° C. for 5 hours, Grignard reagent solution was obtained. Next, 27.0 g (0.3 mol; manufactured by Wako Pure Chemical Industries, Ltd.) of dimethyl carbonate, Toluene 10.8 g (0.3 weight ratio / o-chlorobenzotrifluoride: Wako Pure Chemical Industries, Ltd.) was placed in a 200 ml four-necked flask equipped with a thermometer, While stirring the inside of the system with nitrogen, the mixture was stirred in a water bath. The Grignard reagent solution was added to the reaction solution at a temperature of 40 to 50 ° C. , While controlling the concentration of the solution. The whole amount of the Grignard reagent solution was added dropwise, followed by stirring at 50 ° C. for 7 hours. After completion of stirring, the reaction solution was cooled to room temperature, and in a water bath, 39.2 g of a 3% hydrogen chloride aqueous solution was gradually added dropwise. After the dropwise addition, the mixture was stirred for 1 hour to complete the hydrolysis. After hydrolysis, stirring was stopped, and by static separation liquid, An oil phase containing methyl 2- (trifluoromethyl) benzoate was obtained. As a result of analysis of the obtained oil phase by gas chromatography (GC) The reaction yield of methyl 2- (trifluoromethyl) benzoate is It was 80.7% (based on raw material o-chlorobenzotrifluoride).
References:
Toray Fine Chemicals Co., Ltd.;Nakatani, Jiro;Nozoe, Tatsuhiro JP2016/69299, 2016, A Location in patent:Paragraph 0045-0048
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
81290-20-2
410 suppliers
$13.00/1g

344-96-7
124 suppliers
$10.00/1g
201230-82-2
1 suppliers
inquiry
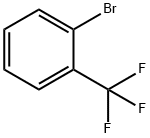
392-83-6
329 suppliers
$6.00/10g

344-96-7
124 suppliers
$10.00/1g
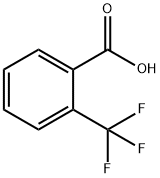
433-97-6
334 suppliers
$10.00/5g