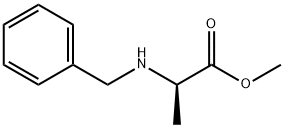
methyl (2R)-2-(benzylamino)propanoate synthesis
- Product Name:methyl (2R)-2-(benzylamino)propanoate
- CAS Number:120571-58-6
- Molecular formula:C11H15NO2
- Molecular Weight:193.24

14316-06-4
345 suppliers
$11.00/10g

100-52-7
945 suppliers
$21.30/2g

120571-58-6
11 suppliers
inquiry
Yield:120571-58-6 91%
Reaction Conditions:
Stage #1: (R)-2-amino-propionic acid methyl ester hydrochloride;benzaldehydewith triethylamine in tetrahydrofuran at 20; for 48 h;
Stage #2: with sodium tetrahydridoborate in methanol at 0; for 3 h;
Steps:
1.A
Example 1 (2R, 5S)-4- (8-Cyano-quinolin-5-yl)-2, 5-dimethyl-piperazine-l-carboxylic acid (4- trifluoromethyl-pyridin-3-yl)-amide A. Preparation of (2R)-2-Benzylamino-propionic acid methyl ester (1A) Benzaldehyde (20 ml, 0.2 mol) and TEA (25 ml, 0.18 mol) were added to D- alanine methyl ester hydrochloride (25g, 0.18 mol) in THF (300 ml) at RT. After 48 hrs, the reaction mixture was filtered through celite (the mixture was washed with 150 ml THF) and concentrated. The reaction crude was dissolved in MeOH (400 ml) and cooled to 0°C. Sodium borohydride (7.5 g, 0.2 mol) was slowly added in portions, and the reaction mixture was stirred at 0°C for 3 hrs. The reaction was quenched with 1N NaOH (125 ml), concentrated and extracted with DCM (4 x 200 ml), dried over Na2S04, and concentrated to isolate 1A as a clear, off-white oil (31.7 g, 91%). [M+H] + = 194. B. Preparation of (3S, 6R)-l-Benzyl-3, 6-dimethyl-piperazine-2, 5-dione (1B) (2R) -2-Benzylamino-propionic acid methyl ester (1A) (1.0 g, 5.2 mmol) and N-tert-butoxycarbonyl-L-alanine (0.98 g, 5.2 mmol) were added to DCC (1.07 g, 5.2 mmol) in DCM (55 ml) at 0°C. After addition, the reaction mixture was warmed to RT, stirred for 24 hrs, filtered through celite (with 2 x 50 ml diethyl ether wash), and concentrated. The reaction mixture was dissolved in DCM (30 ml), cooled to 0°C, and TFA (5 ml) was added. After 10 min, the reaction mixture was warmed to RT and stirred for 3 hrs. The reaction was quenched by slow addition of saturated NaHCO3 (100 ml), and extracted with DCM (3 x 75 ml), dried over Na2S04 and purified by silica gel flash chromatography (EtOAc) to isolate 1B as a clear oil (0.68 g, 57%). [M+H] + = 233.14. C. Preparation of (2S, 5R)-1-Benzyl-2, 5-dimethyl-piperazine (1C) LiAlH4 (60 mmol, 60 ml of 1.0 M solution in THF) was added to (3S, 6R)-1- benzyl-3,6-dimethyl-piperazine-2, 5-dione (1B) (3.48 g, 15 mmol) in THF (100 ml) at 0°C. After addition, the reaction mixture was heated at 70 °C for 24 hrs. The reaction was cooled to 0 °C and quenched by slow addition of H20 (3.5 ml), 1 N NaOH (3.5 ml) and H2O (3.5 ml). The reaction mixture was filtered through celite and washed with THF (100 ml) and EtOAc (100 ml), dried over Na2S04, concentrated and purified by flash chromatography (15% MeOH/CHCl3 with 1% TEA) to isolate 1C as a clear oil (2.43 g, 79%). [M+H] + = 205.16. D. Preparation of 5-Bromo-quinoline-8-carbonitrile (1D) NaN02 (345 mg, 5.0 mmol) in H20 (2.0 ml) was added to 5-amino-quinoline- 8-carbonitrile (770 mg, 4.6 mmol) in 48% HBr (aqueous, 2.0 ml) at 0 °C. After 30 minutes, CuBr (522 mg, 3.6 mmol) in 48% HBr (aqueous, 1.5 ml) is added. After addition, the reaction mixture was heated at 100 °C for 1 hr and cooled to RT. The reaction was neutralized to pH 8 with 1N NaOH and extracted with EtOAc (2X100 ml). The pooled organic phase was washed with H20 (100 ml), saturated NH40H (100 ml), dried over Na2S04, concentrated, and purified by silica gel flash chromatography (stepwise gradient: DCM to 2% EtOAc/DCM) to isolate compound 1D as a white solid (550 mg, 51%). [M+H] +=235. 09. E. Preparation of (2S, 5R)-5- (4-Benzyl-2, 5-dimethyl-piperazin-1-yl)- quinoline-8-carbonitrile (lE) In a microwave compatible reaction flask, (+)-(S)-N, N-Dimethyl-1-[(R)-2- (diphenylphosphino) ferrocenyl] ethylamin (15.4 mg, 0.035 mmol), compound 1D (82 mg, 0.35 mmol), and compound 1C (86 mg, 0.42 mmol) were dissolved in toluene (3.5 ml) and degassed with N2 for 5 min. Tris (dibenzylideneacetone) dipalladium (0) (32 mg, 0.035 mmol), sodium tert-butoxide (50 mg, 0.52 mmol) were added, and the reaction mixture was degassed with N2 for 5 additional min. The reaction mixture was heated at 120 °C under high absorption microwave for 40 minutes, diluted with EtOAc (2 ml), filtered, concentrated, and purified using prep HPLC to isolate compound 1E as a TFA salt. Compound 1E was diluted in saturated aqueous NaHCO3 (10 ml) and extracted with DCM (2X10ml), and concentrated to isolate 1E as yellow film (17.8 mg, 14%). [M+H] + = 357. 48. F. Preparation of (2S, 5R)- 5- (2, 5-Dimethyl-piperazin-1-yl)-quinoline-8- carbonitrile (1F) 1-Chloroethyl chloroformate (0.054 ml, 0.5 mmol) was added to compound 1E (18 mg, 0.05 mmol) in dichloroethane (1 ml). The reaction mixture was heated at 85 °C for 18 hrs, concentrated and dissolved in MeOH and heated at 65 °C for additional 24 hrs. The reaction mixture was diluted in saturated aqueous NaHCO3 (10 ml) and extracted with DCM (2x10ml), dried over Na2S04, concentrated and purified by silica gel flash chromatography (10% MeOH/CHCl3 with 1% TEA) to isolate compound IF as yellow film (4.5 mg, 34%). [M+H] + = 267.36 G. Preparation of (2R, 5S)-4- (8-Cyano-quinolin-5-yl)-2, 5-dimethyl- piperazine-1-carboxylic acid (4-trifluoromethyl-pyridin-3-yl) -amide) (1) 4-Trifluoromethyl-pyridin-3-ylamine (4 mg, 0.025 mmol) and TEA (0.0035 ml, 0.025 mmol) in DCM (0.5 ml) were added to triphosgene (2.5 mg, 0.0085 mmol) in DCM (0.25 ml) at 0°C. After 5 min the reaction mixture was warmed to RT. Compound 1F (4.5 mg, 0.017 mmol) and TEA (0.0035 ml, 0.025 mmol) in DCM (0.5 ml) were added. After 30 minutes, the reaction mixture was concentrated and purified by silica gel flash chromatography (5% MeOH/CHCIa with 1% TEA) to isolate Example 1 ((2R, 5S)-4-(8-Cyano-quinolin-5-yl)-2, 5-dimethyl-piperazine-1-carboxylic acid (4-trifluoromethyl-pyridin-3-yl) -amide) as a clear film (1.2 mg, 16%). HPLC: 92.9% at 1.45 min. (retention time) (Phenomenex S5 ODS column, 4.6 x 30 mm, eluting with 10-90% aqueous methanol over 2 min containing 0. 1 % TFA, 5 mL/min, monitoring at 220 nm). MS (ES): m/z 455.37 [M+H] +.
References:
WO2005/40136,2005,A1 Location in patent:Page/Page column 77-78

14316-06-4
345 suppliers
$11.00/10g

100-52-7
945 suppliers
$21.30/2g

120571-58-6
11 suppliers
inquiry
188798-80-3
1 suppliers
inquiry

600-22-6
367 suppliers
$6.00/5g

100-46-9
463 suppliers
$5.00/5G

120571-58-6
11 suppliers
inquiry
31022-10-3
29 suppliers
inquiry

100-39-0
425 suppliers
$10.00/10g

14316-06-4
345 suppliers
$11.00/10g

120571-58-6
11 suppliers
inquiry

67-56-1
739 suppliers
$9.00/25ml

100-46-9
463 suppliers
$5.00/5G
167823-32-7
0 suppliers
inquiry

120571-58-6
11 suppliers
inquiry

31022-10-3
29 suppliers
inquiry