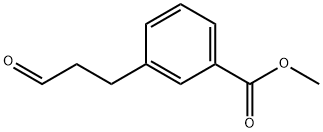
METHYL 3-(3-OXOPROPYL)BENZOATE synthesis
- Product Name:METHYL 3-(3-OXOPROPYL)BENZOATE
- CAS Number:111393-29-4
- Molecular formula:C11H12O3
- Molecular Weight:192.21
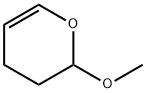
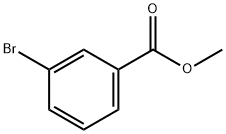
4454-05-1
137 suppliers
$9.00/25g
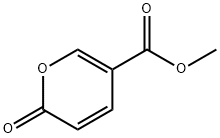
6018-41-3
168 suppliers
$6.00/250mg

111393-29-4
21 suppliers
inquiry
Yield: 77%
Reaction Conditions:
in toluene at 200; for 16 h;Inert atmosphere;
Steps:
General Procedure for the Diels-Alder Reaction of Methyl Coumalate
General procedure: The synthesis of 3c is representative, with the exception of vinyl ethers 2a and 2b where 5.0 equivalents are required to solely obtain the protected alcohols 3a and 3b, respectively. To a sealable 25-mL pressure vessel was successively added 1 (0.154 g, 1.0 mmol), toluene (2 mL), and 2c (0.33 mL, 3.0 mmol) under argon. The solution was heated to 200 oC and stirred for 16 h. Upon completion of the reaction, the sealable pressure vessel was cooled to room temperature. The solution was transferred to another flask, while rinsing with ethyl acetate, after which the solution was concentrated in vacuo. The crude product was purified by flash column chromatography (silica gel, EtOAc:hexanes 1:20) to afford 3c (0.18 g, 77% yield aspale yellow needles
References:
Lee, Jennifer J.;Kraus, George A. [Tetrahedron Letters,2013,vol. 54,# 19,p. 2366 - 2368] Location in patent:supporting information
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

111393-29-4
21 suppliers
inquiry
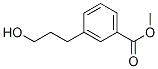
85431-06-7
7 suppliers
inquiry

111393-29-4
21 suppliers
inquiry
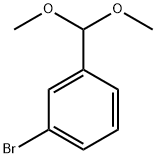
67073-72-7
25 suppliers
inquiry

111393-29-4
21 suppliers
inquiry