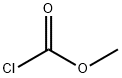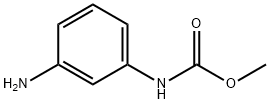
methyl (3-aminophenyl)carbamate(SALTDATA: HCl) synthesis
- Product Name:methyl (3-aminophenyl)carbamate(SALTDATA: HCl)
- CAS Number:6464-98-8
- Molecular formula:C8H10N2O2
- Molecular Weight:166.18
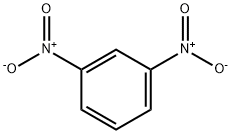
99-65-0
4 suppliers
$32.00/25g
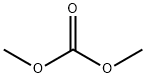
616-38-6
663 suppliers
$5.00/5G
4930-04-5
1 suppliers
inquiry

6464-98-8
8 suppliers
inquiry
Yield:4930-04-5 60% ,6464-98-8 20%
Reaction Conditions:
with hydrogen;1 wt% Au/CeO2 at 150; under 11251.1 Torr; for 23 h;Inert atmosphere;
Steps:
4
In a 5 ml reinforced glass flask capable of pressure sealing dimethyl carbonate (2 ml), meta-dinitrobenzene (150 mg), the Au/CeO2 catalyst (100 mg, 1 wt% gold relative to titanium) are placed. A magnetic stirrer is added and it is proceeded to close the reactor. After purging with N2 for 5 minutes, the reactor is loaded with hydrogen gas at a pressure of 15 bars. The reactor is immersed in a silicone bath preheated at 150° C and the mixture is magnetically stirred for 23 hrs, After this time, the reactor is brought to atmospheric pressure and opened. The catalyst is filtered and the liquid phase is analyzed by gas chromatography. The formation of 1,3-bis(methoxycarbonylamino) benzene in a 60% yield Is observed. Smaller amounts (20%) of mono carbamoylate 1,3-diaminobenzene are detected.
References:
EP2407449,2012,A1 Location in patent:Page/Page column 5