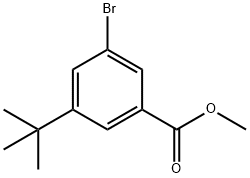
METHYL-3-TERT-BUTYL-5-BROMO-BENZOATE synthesis
- Product Name:METHYL-3-TERT-BUTYL-5-BROMO-BENZOATE
- CAS Number:560131-64-8
- Molecular formula:C12H15BrO2
- Molecular Weight:271.15

560131-64-8
40 suppliers
$283.00/1g

7498-54-6
82 suppliers
$21.00/100mg
Yield:7498-54-6 81%
Reaction Conditions:
Stage #1: methyl 3-bromo-5-tert-butylbenzoatewith sodium hydroxide;hydrogen;palladium(II) hydroxide/carbon in methanol;water; under 2585.81 Torr; for 22 h;
Stage #2: with hydrogenchloride in water;
Stage #3: with hydrogenchloride;palladium 10% on activated carbon;palladium(II) hydroxide/carbonmore than 3 stages;
Steps:
C2.1
A mixture of the commercially available methyl 3-bromo-5-tert-butylbenzoate (700 mg, 2.58 mMol), aqueous NaOH (1 N, 7.75 mL, 7.75 mMol), and Pearlman's catalyst (100 mg) in methanol (20 mL) was hydrogenated at 50 psi for 22 hours. The catalyst was removed by filtration and rinsed with a small amount of methanol. The filtrate was concentrated in-vacuo to remove methanol, and the aqueous mixture was acidified with 1 N HCl (10 mL), then extracted with ethyl acetate (3*20 mL). The combined organic phases were dried over sodium sulfate, then concentrated in-vacuo. Analysis of the resulting material by LC/MS showed that the ester had hydrolyzed to the carboxylic acid, but that the bromide was still present. The material was dissolved in methanol (20 mL), and hydrogenated overnight at 50 psi in the presence of 1 N aqueous NaOH (5.2 mL, 5.2 mMol) and 10% palladium on activated carbon (50 mg). Analysis of the crude reaction mixture by LC/MS showed that the bromine was still present, so Pearlman's catalyst (200 mg) was added, and hydrogenation at 50 psi was continued for 23 hours. MS showed that the reaction was now complete, so the reaction was worked up as described previously in this example to yield 376 mg (81% yield) of white powder as product. MS (AP-)=177 (M-H)
References:
US2005/54627,2005,A1 Location in patent:Page/Page column 39

560131-64-8
40 suppliers
$283.00/1g

746671-59-0
0 suppliers
inquiry