
Methyl 4-chloro-3-(trifluoromethoxy)benzoate synthesis
- Product Name:Methyl 4-chloro-3-(trifluoromethoxy)benzoate
- CAS Number:1261444-00-1
- Molecular formula:C9H6ClF3O3
- Molecular Weight:254.59
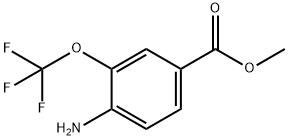
457097-93-7
46 suppliers
inquiry

1261444-00-1
7 suppliers
inquiry
Yield:1261444-00-1 55%
Reaction Conditions:
Stage #1: 4-amino-3-trifluoromethoxybenzoic acid methyl esterwith hydrogenchloride;water;sodium nitrite in acetonitrile at 0; for 0.666667 h;
Stage #2: with copper(l) chloride in acetonitrile at 0; for 0.25 h;Inert atmosphere;
Steps:
38.2 Step 2: Methyl 4-chloro-3-(trifluoromethoxy)benzoate
To a solution of methyl 4-amino-3-(trifluoromethoxy)benzoate (5 g, 25.33 mmol) in CH3CN (100 mL) was added concentrated HC1 (20 mL) in an ice/water bath. This was followed by the addition of a solution of NaN02 (5.9 g, 85.51 mmol) in water (20 mL) dropwise at 0-5°C. The reaction was stirred for 40 min at 0°C in an ice/water bath. To this was added copper chloride (5.9 g, 59.60 mmol) in portions at 0°C. The resulting solution was stirred for 1.5 min at 0°C in a water/ice bath maintained under an inert atmosphere of nitrogen. The resulting solution was extracted with ethyl acetate (3 x 100 mL) and the organic layers combined and dried in an oven under reduced pressure. The resulting mixture was concentrated in vacuo. The residue was purified by silica gel column chromatography with ethyl acetate/petroleum ether (1 : 100) to afford methyl 4-chloro-3- (trifluoromethoxy)benzoate as yellow oil (3 g, 55%).LC/MS (ES, m/z) [M+H]+ 255.0 *H NMR (300 MHz, DMSO) 57.91 - 7.99 (m, 2H), 7.56 (d, J (s, 3H)
References:
WO2014/66795,2014,A1 Location in patent:Paragraph 0175
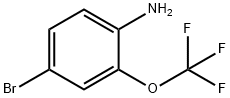
175278-09-8
195 suppliers
$7.00/5g

1261444-00-1
7 suppliers
inquiry