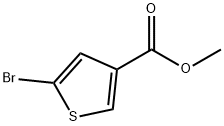
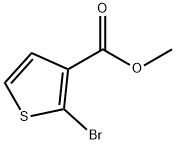
Methyl 5-broMothiophene-3-carboxylate synthesis
- Product Name:Methyl 5-broMothiophene-3-carboxylate
- CAS Number:88770-19-8
- Molecular formula:C6H5BrO2S
- Molecular Weight:221.07

67-56-1
736 suppliers
$7.29/5ml-f
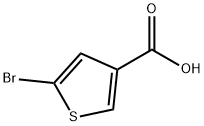
100523-84-0
171 suppliers
$8.00/250mg

88770-19-8
75 suppliers
$31.00/250mg
Yield: 91%
Reaction Conditions:
Stage #1:methanol;2-bromothiophene-4-carboxylic acid with sulfuric acid for 12 h;Reflux;
Stage #2: with sodium hydrogencarbonate in water
Steps:
59; 60
A solution of bromine (2 mL, 39.06 mmol) in glacial acetic acid (64 mL) was added slowly to a stirred solution of Int-1 (5 g, 39.06 mmol) in glacial acetic acid (37 mL) at 0° C. and stirred at room temperature for 20 minutes. The reaction mixture was then poured into ice water while stirring vigorously. The white precipitated solid was filtered, washed with water and recrystallised from hot water to afford pure Int-2 as white solid (3.68 g, 46%). 1H NMR (200 MHz, dmso-d6): δ 7.55 (s, 1H), 8.17 (s, 1H). To a stirred solution of Int-2 (3.4 g, 16.42 mmol) in methanol (40 mL) was added H2SO4 (321 mg, 3.28 mmol) and stirred under reflux for 12 hours. Volatiles from the reaction mixture were distilled off under reduced pressure and the resulting residue was extracted with DCM (75 mL). The organic extract was washed with water (40 mL), saturated aqueous NaHCO3 solution (40 mL), brine (40 mL) and dried over Na2SO4, filtered and concentrated under vacuum to afford pure Int-3 as colorless viscous oil (3.3 g, 91%). 1H NMR (200 MHz, dmso-d6): δ 7.98 (s, 1H), 7.44 (s, 1H), 3.87 (s, 3H). To a stirred solution of Int-3 (0.5 g, 2.26 mmol) in dioxane (25 mL) was added Int-4 (0.51 g, 2.26 mmol) followed by Pd (OAc) 2 (105 mg, 0.45 mmol), xanthpos (265 mg, 0.497 mmol) and CS2CO3 (1.18 g, 3.62 mmol). The reaction mixture was degassed under vacuum, bubbled with N2 for 10 minutes and stirred under reflux for 20 hours. The reaction mixture was concentrated under reduced pressure and was purified by column chromatography to isolate product and a very close spot together eluting with EtOAc. This mixture (140 mg) was further purified with preparative HPLC to obtain pure Int-5 as a yellow solid (80 mg, 10%). Mass (m/z): 366.0 [M++1]. 1H NMR (200 MHz, dmso-d6): 9.50 (d, J=7 Hz, 1H), 8.51 (d, J=7 Hz, 1H), 8.56 (d, J=5.2 Hz, 1H), 7.74 (brs, 1H), 7.66 (d, J=11.8 Hz, 1H), 7.35 (d, J=7.4 Hz, 1H), 7.11 (s, 1H), 7.02 (d, J=5.6 Hz, 1H), 6.87 (t, J=6.8 Hz, 1H), 3.86 (s, 3H), 2.73 (s, 3H). To a stirred solution of Int-5 (0.7 g, 1.91 mmol) in THF: MeOH: H2O (2:1:2) (50 ml) was added LiOH (0.24 g, 5.74 mmol) at room temperature and stirred while heating at 50° C. for 16 hours. Volatiles from the reaction mixture were distilled off under reduced pressure, the reaction mixture was acidified to about pH 5-6 with 2N HCl. The precipitated solid was filtered and dried under vacuum to afford pure Int-6 (0.51 g, 76%). Mass (m/z): 352.0 [M++1]. 1H NMR (200 MHz, dmso-d6): δ 12.45 (brs, 1H), 10.87 (s, 1H), 9.70 ((brs, 1H), 8.60 (d, J=4.68 Hz, 1H), 7.67-7.59 (m, 2H), 7.45 (t, J=8.4 Hz, 1H), 7.13 (d, J=5.2 Hz, 1H), 7.04 (s, 2H), 2.65 (s, 3H).
References:
Melvin, JR., Lawrence S.;Graupe, Michael;Venkataramani, Chandrasekar US2010/29638, 2010, A1 Location in patent:Page/Page column 110

100523-84-0
171 suppliers
$8.00/250mg
77-78-1
289 suppliers
$19.69/1ml

88770-19-8
75 suppliers
$31.00/250mg

88-13-1
368 suppliers
$5.00/1g

88770-19-8
75 suppliers
$31.00/250mg
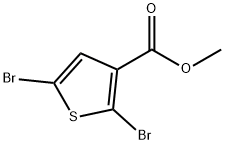
89280-91-1
34 suppliers
inquiry
76360-43-5
90 suppliers
$33.00/250mg

88770-19-8
75 suppliers
$31.00/250mg

100523-84-0
171 suppliers
$8.00/250mg

88770-19-8
75 suppliers
$31.00/250mg