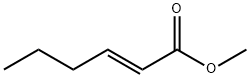
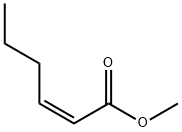
METHYL TRANS-2-HEXENOATE, 98 synthesis
- Product Name:METHYL TRANS-2-HEXENOATE, 98
- CAS Number:13894-63-8
- Molecular formula:C7H12O2
- Molecular Weight:128.17

67-56-1
738 suppliers
$7.29/5ml-f
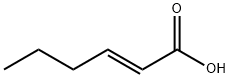
13419-69-7
248 suppliers
$10.00/1g

13894-63-8
66 suppliers
$22.00/25g
Yield: 100%
Reaction Conditions:
Stage #1:(E)-2-Hexenoic acid with oxalyl dichloride in tetrahydrofuran;N,N-dimethyl-formamide for 0.5 h;Cooling with ice;
Stage #2:methanol in tetrahydrofuran;N,N-dimethyl-formamide at 10 - 35; for 2 h;
Steps:
87 Methyl (2E)-hex-2-enoate
Reference Example 87
Methyl (2E)-hex-2-enoate
To a solution (100 mL) of (2E)-hex-2-enoic acid (5.0 g) in tetrahydrofuran were added dropwise under ice-cooling oxalyl chloride (3.76 mL) and N,N-dimethylformamide (1 mL).
The mixture was stirred at the same temperature for 30 min, methanol (10 mL) was gradually added to the reaction mixture, and the mixture was stirred at room temperature for 2 hr.
The solvent was evaporated under reduced pressure, and the residue was treated with 6% aqueous sodium hydrogencarbonate solution, and extracted with diethyl ether.
The extract was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure (50 Torr, water bath 10°C) to give the title compound as a colorless oil (yield 5.67 g, about 100%).
1H-NMR (CDCl3)δ:0.94 (3H, t, J=7.5 Hz), 1.43-1.53 (2H, m), 2.14-2.21 (2H, m), 3.73 (3H, s), 5.82 (1H, dt, J=1.8, 15.6 Hz), 6.97 (1H, dt, J=6.9, 15.6 Hz).
References:
Takeda Pharmaceutical Company Limited;Kajino, Masahiro;Hasuoka, Atsushi;Tarui, Naoki;Takagi, Terufumi EP2336107, 2015, B1 Location in patent:Paragraph 0261

13419-69-7
248 suppliers
$10.00/1g

74-88-4
340 suppliers
$15.00/10g

13894-63-8
66 suppliers
$22.00/25g

123-72-8
403 suppliers
$12.32/25ML
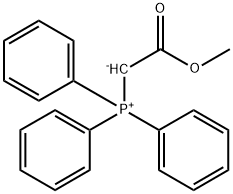
21204-67-1
17 suppliers
inquiry

13894-63-8
66 suppliers
$22.00/25g
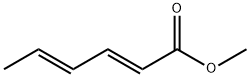
689-89-4
76 suppliers
inquiry

13894-63-8
66 suppliers
$22.00/25g

21204-67-1
17 suppliers
inquiry

71-36-3
1028 suppliers
$14.00/25mL

13894-63-8
66 suppliers
$22.00/25g
13894-64-9
0 suppliers
inquiry