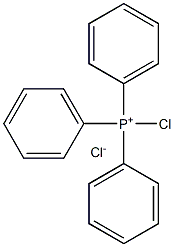
Methyl(triphenyl)phosphonium chloride synthesis
- Product Name:Methyl(triphenyl)phosphonium chloride
- CAS Number:896-33-3
- Molecular formula:C20H20ClP
- Molecular Weight:326.8
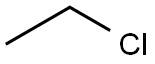
75-00-3
206 suppliers
$29.39/crm40015
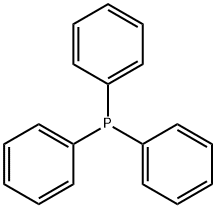
603-35-0
698 suppliers
$5.00/5 g

896-33-3
171 suppliers
$22.39/5G
Yield: 95.23%
Reaction Conditions:
in acetone at 150; under 9000.9 Torr; for 40 h;Autoclave;Solvent;Temperature;Pressure;
Steps:
3 Example 3
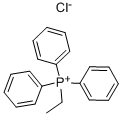
Weigh 1 mol of triphenylphosphine and 10 moles of acetone, put them in a 2000 L pressure reactor,Then add 1.5mol of ethyl chloride, after the completion of sealing the reactor; open the steam inlet valve for heating,Control the pressure inside the reactor temperature of 150 ,The pressure in the kettle was 12 kg / cm2, and the temperature was maintained for 40 hours;And then close the steam intake valve, through the cooling water on the pressure reactor to cool down, down to 60 ,Pressure force reactor vent, holding 1 hour, the recovery row did not respond to the reaction of ethyl chloride, to normal pressure, continueCooling to room temperature; and then the reaction of the reaction product after centrifugal separation, centrifugal supernatantLiquid into the water detection, water content of 4.8%, supernatant left to stand; centrifugal sediment at 105 Drying for 18 hours, and then mixed with acetonitrile, heated to reflux after cooling crystallization, standing centrifugal, centrifuged for 48hAnd the precipitate was the ethyltriphenylphosphonium chloride, and 0.9523 mol of ethyltriphenylphosphonium chloride was weighed,The yield was 95.23%, and the purity was 99.56%.
References:
Kent Catalytic Materials Co., Ltd.;Xiang, Feiyong CN106397483, 2017, A Location in patent:Paragraph 0017; 0018; 0019; 0020; 0021; 0022; 0023-0025
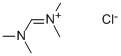
1071-38-1
8 suppliers
$58.50/5g
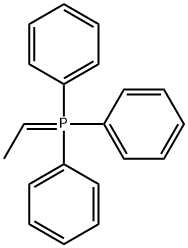
1754-88-7
6 suppliers
inquiry

5762-56-1
67 suppliers
$102.00/1g

896-33-3
171 suppliers
$22.39/5G
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
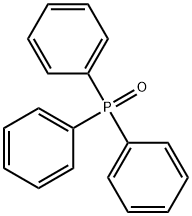
791-28-6
484 suppliers
$6.00/25g

896-33-3
171 suppliers
$22.39/5G