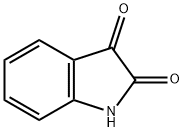
MFCD00435229 synthesis
- Product Name:MFCD00435229
- CAS Number:55618-86-5
- Molecular formula:C15H15NO2
- Molecular Weight:241.29
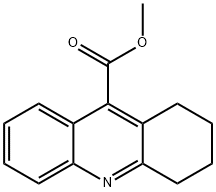
38186-54-8
37 suppliers
$45.00/50mg

74-88-4
341 suppliers
$15.00/10g

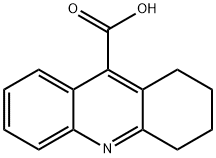
55618-86-5
16 suppliers
inquiry
Yield:55618-86-5 72%
Reaction Conditions:
with potassium carbonate in acetone; for 5 h;Reflux;
Steps:
5.1.2.2. Methyl 6-chloro-2-(4-methoxyphenyl) quinoline-4-carboxylate (3a)
General procedure: 6-Chloro-2-(4-methoxyphenyl) quinoline-4-carboxylic acid (2a) (0.98 g, 3 mmol) and potassium carbonate (2.07 g, 15 mmol) were weighed into a round-bottom flask. Methyl iodide (0.95 mL, 15 mmol) and acetone (4 mL) were added. The reaction mixture was refluxed with magnetic stirring. The progress of the reaction was monitored by TLC. The reaction was completed after 5 h. The solvent was evaporated in vacuo and water was added to the remaining mixture. The product was collected by suction filtration and air-dried. The crude product was separated on a silica gel column to obtain pure 3a at 90% yield.
References:
Wu, Yiran;Chen, Zheng;Liu, Ying;Yu, Lanlan;Zhou, Lu;Yang, Suijia;Lai, Luhua [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 11,p. 3361 - 3366] Location in patent:experimental part