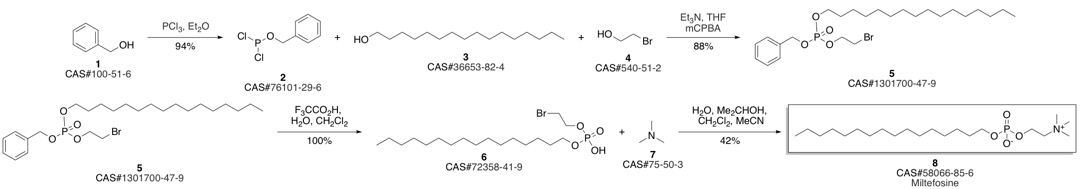
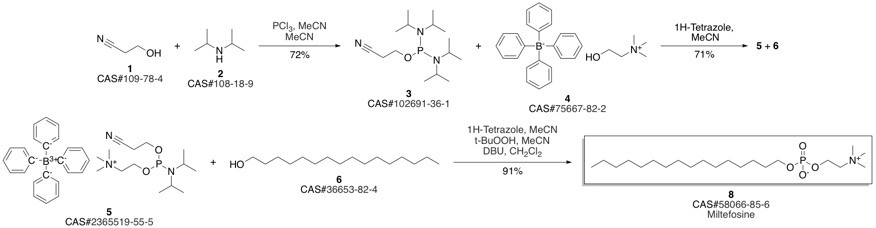
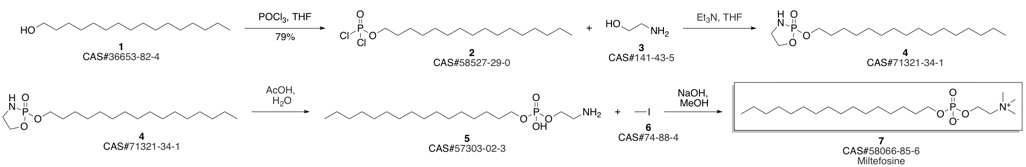
Miltefosine synthesis
- Product Name:Miltefosine
- CAS Number:58066-85-6
- Molecular formula:C21H46NO4P
- Molecular Weight:407.57
Xu, Fangming; Wang, Haibo; Zhao, Jie; Liu, Xiangsheng; Li, Dandan; Chen, Chaojian; Ji, Jian. Chiral Packing of Cholesteryl Group as an Effective Strategy To Get Low Molecular Weight Supramolecular Hydrogels in the Absence of Intermolecular Hydrogen Bond. Macromolecules (Washington, DC, United States). Volume 46. Issue 11. Pages 4235-4246. Journal; Online Computer File. (2013).
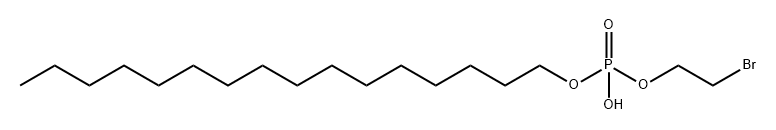
72358-41-9
0 suppliers
inquiry
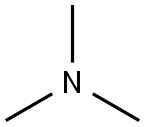
75-50-3
254 suppliers
$15.00/25ml

58066-85-6
280 suppliers
$28.00/50mg
Yield:58066-85-6 81%
Reaction Conditions:
in chloroform;water;isopropyl alcohol;acetonitrile at 70;
Steps:
The previous compound (1.0 g, 2.33 mmol) inCHCl3/CH3CN/i-PrOH 3:5:5 (13 mL) was treated with45% (w/w) aqueous NMe3 (5 mL, 34.3 mmol) at 70°C.When all starting material was consumed (checked byTLC), volatile was removed under vacuum and the residuewas extracted with CHCl3/MeOH 1:3. The organiclayer was washed with sat. NaCl, reduced under vacuum,diluted with CHCl3, dried over MgSO4, filteredand evaporated. The crude residue was purified by flashchromatography (CH2Cl2/MeOH/H2O 75:22:3 to45:45:10) to yield miltefosine (0.77 g, 81%). Rf: 0.1(CH2Cl2/MeOH/H2O 75:22:3). 1H-NMR (400 MHz,MeOD/CDCl3 1:1) δ 0.85 (t, J = 6.2 Hz, 3H); 1.24(m, 26H); 1.61 (tt, J1 = J2 = 6.4 Hz, 2H); 3.31 (s, 9H);3.62 (m, 2H); 3.85 (td, J1 = J2 = 6.5 Hz, 2H); 4.23 (m,2H). 13C-NMR (100.7 MHz, MeOD/CDCl3 1/1) δ14.3; 23.2; 26.4; 30.1 (2C); 30.3 (8C); 31.3; 32.5;54.5; 59. 7 ; 66.7; 67.0. 31P-NMR (162 MHz ;MeOD/CDCl3 1:1) δ - 0.32. IR (ATR) ν 497; 716;746; 853; 927; 957; 1050; 1126; 1246; 1471; 1633;2849; 2914; 3372. HR-MS (ESI+) m/z [M + H]+ calcdfor C21H47NO4P+ 408.3237, found 408.3239.
References:
Gaillard, Boris;Lebeau, Luc;Remy, Jean-Serge;Pons, Fran?oise [Pharmaceutical Research,2020,vol. 37,# 6]
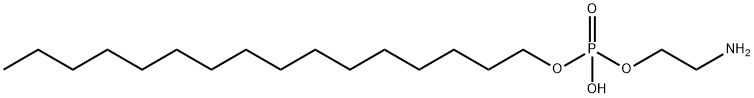
57303-02-3
2 suppliers
inquiry
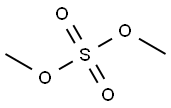
77-78-1
291 suppliers
$22.00/25g

58066-85-6
280 suppliers
$28.00/50mg
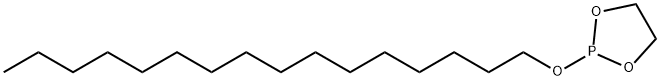
216437-33-1
0 suppliers
inquiry

58066-85-6
280 suppliers
$28.00/50mg
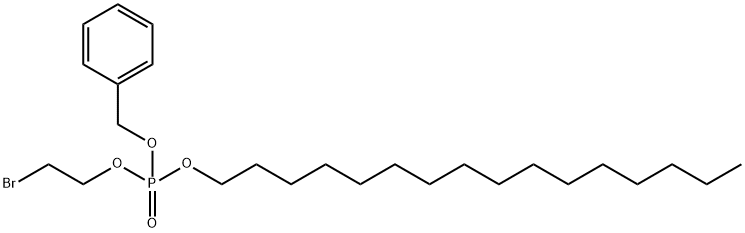
1301700-47-9
0 suppliers
inquiry

58066-85-6
280 suppliers
$28.00/50mg