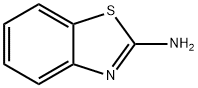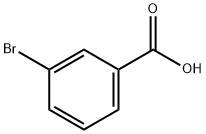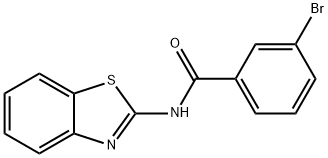
N-1,3-benzothiazol-2-yl-3-bromobenzamide synthesis
- Product Name:N-1,3-benzothiazol-2-yl-3-bromobenzamide
- CAS Number:139233-21-9
- Molecular formula:C14H9BrN2OS
- Molecular Weight:333.2
Yield:139233-21-9 88.6%
Reaction Conditions:
with benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in N,N-dimethyl-formamide at 0 - 20;
Steps:
General procedure for the synthesis of N’-(1,3-benzothiazol-2-yl)-4-substituted benzamides (C1-18)
General procedure: 1,3-Benzothiazole-2-amine (A) (0.242 g, 0.0014 mol) andHOBt (0.450 g, 0.0029 mol) were successively added to thecorresponding aromatic acids (B1-18) (0.2 g, 0.0014 mol) dissolvedin N,N-dimethylformamide (DMF) (15 mL). The mixturewas cooled to 0 °C in an ice bath with stirring, and then,EDCl (0.563 g, 0.00294 mol) was added. The reaction mixturewas then slowly allowed to reach the room temperature over1 h, and then, stirring was further continued at this temperaturetill completion of reaction. Progress of reaction was monitoredwith TLC using n-hexane:ethylacetate (1:2 to 1:6) as eluent.The reaction was quenched by adding saturated NaHCO3solution and then extracted with ethylacetate (20 mL × 3).The organic layer was dried with anhydrous Na2SO4,filteredand concentrated under reduced pressure. The residue thusobtained was purified by column chromatography on silica gelMerck (200 mesh) in glass columns (2 or 3 cm diameter) using 25-30 g of silica gel per one gram of the residue. The elutionof column was started with n-hexane, and then, eluent polaritywas gradually increased with ethyl acetate. Compounds C1-18thus obtained in the yield of 80-95%.
References:
Gurram, Swarupa Rani;Azam, Mohammed Afzal [Chemical Papers,2021,vol. 75,# 10,p. 5435 - 5452]

78225-75-9
0 suppliers
inquiry

139233-21-9
4 suppliers
inquiry