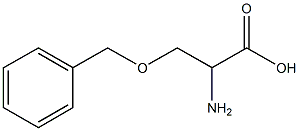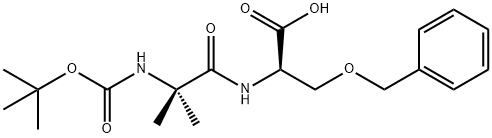
N-2-(3-BENZYLOXY PRIOPIONIC ACID)-2-(N-T-BUTOXYCAR synthesis
- Product Name:N-2-(3-BENZYLOXY PRIOPIONIC ACID)-2-(N-T-BUTOXYCAR
- CAS Number:159634-89-6
- Molecular formula:C19H28N2O6
- Molecular Weight:380.44
Yield:159634-89-6 98.4%
Reaction Conditions:
with triethanolamine;citric acid in tetrahydrofuran;hexane;water;
Steps:
G G.
G. 3-(R)-Benzyloxy-2-(2-tert-butoxycarbonylamino-2-methyl-propionylamino)-propionic acid To a solution of 2-amino-3-benzyloxy-propionic acid (26.2 g, 0.113 mol) in water (101.8 mL) and TEA (28.53 g, 0.282 mol) was added 2-tert-butoxycarbonylamino-2-methyl-propionic acid 2,5-dioxo-pyrrolidin-1-yl ester (33.94 g, 0.113 mol) in THF (407 mL). The mixture was stirred overnight at room temperature under nitrogen. A 10% citric acid solution (500 mL) was added to the mixture. The mixture was stirred for another 10 min., then diluted with ethyl acetate (500 mL). The organic phase was separated from the mixture and washed with water and saturated NaCl solution and then concentrated in vacuo to a thick oil. The crude oil was treated with IPE/hexane (50/50) and cooled to about 10° C. to afford a white solid product (42.3 g, yield 98.4%).
References:
US6448263,2002,B1
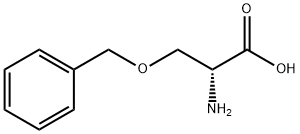
10433-52-0
160 suppliers
$8.00/250mg
![CarbaMic acid, [1-[[(2,5-dioxo-1-pyrrolidinyl)oxy]carbonyl]propyl]-, 1,1-diMethylethyl ester, (S)-](/CAS/GIF/103290-07-9.gif)
103290-07-9
4 suppliers
inquiry

159634-89-6
12 suppliers
inquiry

10433-52-0
160 suppliers
$8.00/250mg

159634-89-6
12 suppliers
inquiry
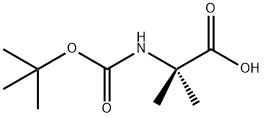
30992-29-1
266 suppliers
$6.00/5g

159634-89-6
12 suppliers
inquiry