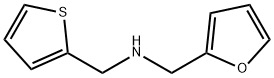
N-(2-FURYLMETHYL)-N-(THIEN-2-YLMETHYL)AMINE synthesis
- Product Name:N-(2-FURYLMETHYL)-N-(THIEN-2-YLMETHYL)AMINE
- CAS Number:90921-60-1
- Molecular formula:C10H11NOS
- Molecular Weight:193.27
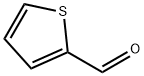
98-03-3
532 suppliers
$5.00/25g
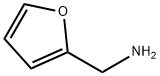
617-89-0
265 suppliers
$16.00/25g

90921-60-1
11 suppliers
$45.00/100mg
Yield:90921-60-1 86%
Reaction Conditions:
Stage #1: thiophene-2-carbaldehyde;furan-2-ylmethanamine in ethanol at 120; for 0.5 h;Microwave irradiation;
Stage #2: with sodium tetrahydroborate;ethanol at 23 - 90; for 19 h;
Steps:
3 1-(furan-2-yl)-N-(thiophen-2-ylmethyl)methanamine
1-(furan-2-yl)-N-(thiophen-2-ylmethyl)methanamine
A mixture containing furan-2-ylmethanamine (1.00 mL, 11.32 mmol) and thiophene-2-carbaldehyde (1.06 mL, 11.32 mmol) in EtOH (22.6 mL) was heated in themicrowave reactor at 120 °C for 0.5 h.
The reaction solution was transferred to a round-bottomed flask, and was then treated with sodium borohydride (0.856 g, 22.63 mmol) at 90 °C for 3 h, then at 23 °C for 16 h. The reaction mixture was concentrated under reduced pressure, and the residue was partitioned between 50 mL of dichloromethane (DCM) and 50 mL of water. The product was extracted with two 25-mL portions of DCM and the combined organic layer was washed with 50 mL of brine, and subsequently dried over anhydrous sodium sulfate (Na2S04). The dried organic layer was concentrated under reduced pressure and purified by silica gel column chromatography(100 g); gradient elution from 90: 10 to 50:50 Hex:EtOAc afforded l-(furan- 2-yl)-N-(thiophen-2-ylmethyl)methanamine (1) as a clear light pale yellow oil; yield: 1.87 g (86 %). LC-MS: t = 1.77 min. 1H NMR (400 MHz, CDCI3) δ 7.38 (dd, J = 1.9, 0.8 Hz, 1H),7.22 (dd, J = 4.8, 1.4 Hz, 1H), 6.98 - 6.92 (m, 2H), 6.32 (dd, J = 3.2, 1.9 Hz, 1H), 6.22 - 6.18 (m, 1H), 3.99 (d, J = 0.7 Hz, 2H), 3.82 (s, 2H), 1.98 (s, 2H). 13C NMR (101 MHz, CDC13) δ 153.5, 143.5, 142.1, 126.8, 125.4, 124.7, 110.3, 107.5, 47.2, 45.0.HRMS (ESI) m/z 194.0635 (M + H)+ (C10Hi2NOS requires 194.0634).
References:
WO2016/4180,2016,A1 Location in patent:Paragraph 00212; 00213; 00214
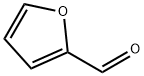
98-01-1
483 suppliers
$22.00/25g
27757-85-3
252 suppliers
$6.00/1g

90921-60-1
11 suppliers
$45.00/100mg