N-(2-Methylbenzyl)butan-1-aMine synthesis
- Product Name:N-(2-Methylbenzyl)butan-1-aMine
- CAS Number:605-09-4
- Molecular formula:C16H12O4
- Molecular Weight:268.26
Yield:605-09-4 380 mg
Reaction Conditions:
Stage #1: nordalberginwith sodium hydroxide in N,N-dimethyl-formamide at 0; for 0.25 h;Inert atmosphere;
Stage #2: methyl iodide in N,N-dimethyl-formamide at 0 - 10; for 3 h;
Steps:
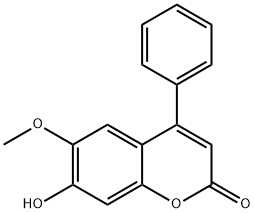
3 Example 3Preparation of 4-phenyl-6-methoxy-7-hydroxycoumarin (I-ac)
Weigh 4-phenyl-6,7-dihydroxy coumarin 500mg in a three-necked flask, argon under the protection of N, N-dimethylformamide 5mL dissolved sample, add ice water bath,The temperature of the reaction system was lowered to 0 ° C,Adding 360 mg of sodium hydroxide,After fully reacting for 15 min,1 μl of methyl iodide was gradually added dropwise to maintain the reaction temperature of the system at 0 to 10 ° C. After the reaction was carried out for 3 hours, the reaction solution was added dropwise to 40 mL of ice water with stirring, and the pH was adjusted to 3 to 4 , There are precipitation, 30min after the filter, collecting filter cake, that is, crude products. The crude product was recrystallized from methanol, heated to reflux for 1 h, cooled and filtered. The filter cake was collected and dried in vacuo to give 380 mg of a white solid.
References:
CN106518825,2017,A Location in patent:Paragraph 0034; 0035; 0036
2555-30-8
57 suppliers
$43.00/1g

605-09-4
0 suppliers
inquiry

482-82-6
89 suppliers
$45.00/10mg

605-09-4
0 suppliers
inquiry
110876-08-9
3 suppliers
inquiry

605-09-4
0 suppliers
inquiry
