
N-(4-FLUOROBENZYL)CYCLOHEXANAMINE synthesis
- Product Name:N-(4-FLUOROBENZYL)CYCLOHEXANAMINE
- CAS Number:356531-67-4
- Molecular formula:C13H18FN
- Molecular Weight:207.29
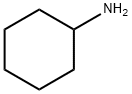
108-91-8
607 suppliers
$10.00/5mL

616-24-0
145 suppliers
$9.00/5g

356531-67-4
19 suppliers
$46.79/250mg
Yield:356531-67-4 100%
Reaction Conditions:
Stage #1: cyclohexylamine;3-aminopentane in methanol at 64; for 3 h;Borch Reduction;
Stage #2: with sodium cyanoborohydride in methanol;ethanol at 20 - 64; for 5 h;Borch Reduction;
Steps:
1
General procedure: General procedure: To generate Compound 1, cyclohexylamine (0.5M in MeOH) was combined with benzaldehyde (0.5M in MeOH) and stirred for 3h at 64°C. The mixture was cooled to rt, followed by two additions of sodium cyanoborohydride (0.5M in EtOH), each followed by stirring at rt for 30m. The mixture was then heated to 64°C for 6h. The reaction mixture was worked-up with water and extracted three times with CH2Cl2. The organic fractions were pooled, dried with magnesium sulfate, and reduced in vacuo. Thesecondary amine was then solubilized in dry CH2Cl2 and combined with 4-chloro-benzoylchloride. Equivalents were based upon the assumption that the secondary amine was formed in 100% yield. Dimethylaminopyridine (0.1equiv) was solubilized in dry CH2Cl2 and added directly to the stirring solution of secondary amine, followed by addition of diisopropylethylamine (1.1equiv). The reaction was then capped, purged with nitrogen gas, and stirred at rt overnight. The reaction mixture was then reduced in vacuo to an oil, which was resolubilized and purified using reverse phase preparative HPLC (isocratic elution: 75% acetonitrile, 25% water). Following preparative HPLC, compound purity was determined using reverse phase analytical HPLC. Compounds were purified to an average purity of greater than 95% (supplementary Table 1).
References:
Ross, Nathan T.;Deane, Rashid;Perry, Sheldon;Miller, Benjamin L. [Tetrahedron,2013,vol. 69,# 36,p. 7653 - 7658]

459-56-3
305 suppliers
$6.00/5g

108-91-8
607 suppliers
$10.00/5mL

356531-67-4
19 suppliers
$46.79/250mg

108-94-1
543 suppliers
$12.00/50g
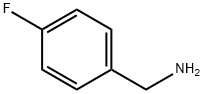
140-75-0
469 suppliers
$10.00/10g

356531-67-4
19 suppliers
$46.79/250mg

108-91-8
607 suppliers
$10.00/5mL

140-75-0
469 suppliers
$10.00/10g

356531-67-4
19 suppliers
$46.79/250mg

108-91-8
607 suppliers
$10.00/5mL

356531-67-4
19 suppliers
$46.79/250mg