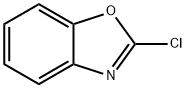
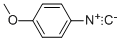
N-(4-Methoxyphenyl)benzo[d]oxazol-2-aMine synthesis
- Product Name:N-(4-Methoxyphenyl)benzo[d]oxazol-2-aMine
- CAS Number:92148-95-3
- Molecular formula:C14H12N2O2
- Molecular Weight:240.26
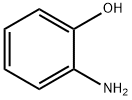
95-55-6
545 suppliers
$5.00/5G
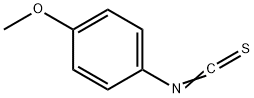
2284-20-0
137 suppliers
$12.00/1g
![N-(4-Methoxyphenyl)benzo[d]oxazol-2-aMine](/CAS/20150408/GIF/92148-95-3.gif)
92148-95-3
5 suppliers
inquiry

17356-08-0
3 suppliers
inquiry
Yield:92148-95-3 136%
Reaction Conditions:
in tetrahydrofuran;
Steps:
16 N2-(4-Methoxyphenyl)-1,3-benzoxazol-2-amine
EXAMPLE 16 N2-(4-Methoxyphenyl)-1,3-benzoxazol-2-amine 4-Methoxyphenyl isothiocyanate (0.321 mL, 2.29 mmol) was added to a solution of 2-amino-phenol (0.25 g, 2.29 mmol) in THF (15 mL) and the reaction was stirred at room temperature for 2 hours when the formation of the thiourea was complete (RP-HPLC Rt 6.388 min, (5% to 95% acetonitrile/0.1M aqueous ammonium acetate, buffered to pH 4.5, over 10 min at 1 mL/min; λ=254 nm; Waters Deltapak C18, 300 ?, 5 μm, 150*3.9 mm column). Anhydrous iron (III) chloride (0.409 g, 2.52 mmol) and triethylamine (0.30 mL, 2.29 mmol) were added, and the mixture was stirred at room temperature for 24 h. The resulting suspension was filtered through celite and the filtrate was concentrated to dryness. The residue was re-dissolved in dichloromethane (100 mL) and washed with sat. aq. CaCl2 (3*100 mL). The organic layer was dried over anhydrous MgSO4 and concentrated in vacuo to afford a brown solid (750 mg, 136%).
References:
US2003/109714,2003,A1

75-15-0
253 suppliers
$40.00/100g
104-94-9
490 suppliers
$9.00/1g

95-55-6
545 suppliers
$5.00/5G
![N-(4-Methoxyphenyl)benzo[d]oxazol-2-aMine](/CAS/20150408/GIF/92148-95-3.gif)
92148-95-3
5 suppliers
inquiry