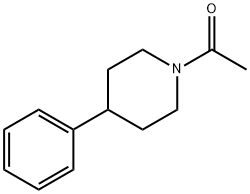
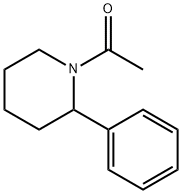
N-acetyl-4-phenylpiperidine synthesis
- Product Name:N-acetyl-4-phenylpiperidine
- CAS Number:32245-87-7
- Molecular formula:C13H17NO
- Molecular Weight:203.28
Yield:32245-87-7 98%
Reaction Conditions:
with pyridine in tetrahydrofuran at 0 - 25; for 14.1667 h;Inert atmosphere;
Steps:
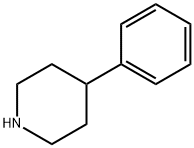
1.9 1-(4-Phenylpiperidin-1-yl)ethanone
Production Example 1-9
1-(4-Phenylpiperidin-1-yl)ethanone
A mixture of commercially available 4-phenylpiperidine (10 g, 62 mmol), pyridine (5.7 mL, 70.5 mmol), and tetrahydrofuran (80 mL) was stirred at 0° C. and a mixture of acetyl chloride (5 mL, 70.3 mmol) and tetrahydrofuran (20 mL) was dripped over 10 minutes.
The mixture was stirred under nitrogen atmosphere at 25° C. for 14 hours.
Ethyl acetate (100 mL) and water (100 mL) were added to the reaction liquid for separation.
The aqueous layer was extracted with ethyl acetate (100 mL), then the organic layers were combined, and the resultant was washed with a saturated aqueous sodium bicarbonate solution (100 mL), water (100 mL), and then a saturated saline solution (50 mL).
The organic layer was dried over anhydrous magnesium sulfate and then the solvent was evaporated to obtain the title compound (12.3 g, 98%).
1H-NMR Spectrum (CDCl3) δ (ppm): 1.52-1.78 (2H, m), 1.81-1.99 (2H, m), 2.14 (3H, s), 2.63 (1H, td, J=12.9, 2.7 Hz), 2.74 (1H, tt, J=12.1, 3.7 Hz), 3.17 (1H, td, J=13.2, 2.6 Hz), 3.84-4.02 (1H, m), 4.69-4.89 (1H, m), 7.08-7.43 (5H, m).
References:
US2014/235614,2014,A1 Location in patent:Paragraph 0279; 0280; 0291
110-86-1
847 suppliers
$9.00/5g
100-59-4
261 suppliers
$42.00/0.25mole

75-36-5
568 suppliers
$17.92/100G

32245-87-7
1 suppliers
inquiry
32245-98-0
0 suppliers
inquiry