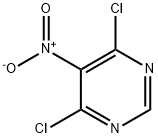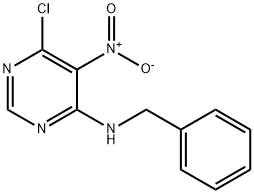
N-benzyl-6-chloro-5-nitro-4-pyrimidinamine synthesis
- Product Name:N-benzyl-6-chloro-5-nitro-4-pyrimidinamine
- CAS Number:54413-44-4
- Molecular formula:C11H9ClN4O2
- Molecular Weight:264.67
Yield:54413-44-4 73%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in ethanol;Inert atmosphere;Autoclave;regioselective reaction;
Steps:
General procedure for C-N bond forming reaction
General procedure: To a stirred solution of chloro nitro pyridine / pyrimidine (1.0 equiv) in EtOH (0.5 mL per 0.52 mmol) was added DIPEA (3.0 equiv) followed by amine (1.05 equiv) at 0oC. The reaction mixture was stirred at 60-80oC for 3-6 h (the reaction was monitored by TLC). After completion of the reaction EtOH was distilled-off under a reduced pressure. The residue was dissolved in EtOAc (50 mL per 1g of crude). The organic layer was washed with water (20 mL), and brine (20 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The resultant crude compound was purified by silica gel column chromatography (100-200 mesh) using 10-20 % ethyl acetate - hexane to afford the desired compound (yield: 85-95 %).
References:
Marepu, Nagaraju;Yeturu, Sunandamma;Pal, Manojit [Bioorganic and Medicinal Chemistry Letters,2018,vol. 28,# 20,p. 3302 - 3306] Location in patent:supporting information