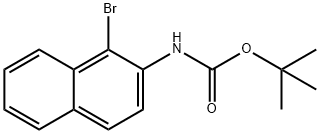
N-Boc-1-bromo-2-naphthalenamine synthesis
- Product Name:N-Boc-1-bromo-2-naphthalenamine
- CAS Number:454713-47-4
- Molecular formula:C15H16BrNO2
- Molecular Weight:322.2
454713-45-2
15 suppliers
$130.00/2.5g

454713-47-4
17 suppliers
inquiry
Yield:454713-47-4 100%
Reaction Conditions:
with N-Bromosuccinimide in acetonitrile at 0; for 2.75 h;
Steps:
2.2 4.2.2. tert-Butyl 1-bromo-2-naphthylcarbamate (12)
A stirred solution of 11 (92?g, 0.38?mol) in acetonitrile (680?mL) at 0?°C was treated portionwise over 2?h with solid NBS (81?g, 0.45?mol). After the addition was complete the reaction mixture was stirred for a further 45?min?at 0?°C. To the mixture was added a cold solution of aqueous NaHCO3 (0.2N, 2?L) and the mixture was stirred at 0?°C for 1?h. The solid was filtered off and washed with water (5?*?200?mL), then dried in vacuum over solid KOH overnight to give 12 as a beige solid (121?g, 100%); mp 88-89 [lit. mp 88-89?°C [19]; 90-91?°C (MeOH) [3]]; δH [(CD3)2SO] 8.78 (s, 1H), 8.15 (d, J?=?8.6?Hz, 1H), 7.99-7.92 (m, 2H), 7.71 (d, J?=?8.8?Hz, 1H), 7.69-7.64 (m, 1H), 7.58-7.53 (m, 1H), 1.49 (s, 9H); consistent with that reported [3].
References:
Lee, Ho H.;Dickson, Benjamin D.;Stevenson, Ralph J.;Yang, Shangjin;Tercel, Moana [Tetrahedron,2019,vol. 75,# 22,p. 3001 - 3007]
454713-46-3
0 suppliers
inquiry

454713-47-4
17 suppliers
inquiry

24424-99-5
821 suppliers
$13.50/25G

454713-47-4
17 suppliers
inquiry

20191-75-7
93 suppliers
inquiry

454713-47-4
17 suppliers
inquiry
93-09-4
295 suppliers
$5.00/1g

454713-47-4
17 suppliers
inquiry