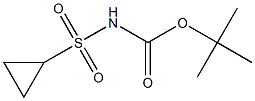
N-BOC-1-METHYLCYCLOPROPANE-1-SULFONAMIDE synthesis
- Product Name:N-BOC-1-METHYLCYCLOPROPANE-1-SULFONAMIDE
- CAS Number:849022-29-3
- Molecular formula:C9H17NO4S
- Molecular Weight:235.3
Yield:849022-29-3 81%
Reaction Conditions:
Stage #1: tert-butyl (cyclopropylsulfonyl)carbamatewith n-butyllithium in tetrahydrofuran;hexane at -78 - 20; for 2.5 h;
Stage #2: methyl iodide in tetrahydrofuran;hexane at -78 - 20;
Steps:
2 Preparation of tert-butyl(1-methylcyclopropyl) sulfonylcarbamate
A solution of tert-butyl cyclopropylsulfonylcarbamate (4.3 g, 20 mmol) was dissolved in dry THF (100 ml) and cooled to -78° C. To this solution was added n-BuLi (17.6 ml, 44 mmol, 2.5 M in hexane) slowly. The reaction mixture was allowed to warm to room temperature over a period of 1.5 h. This mixture was then cooled to -78° C., and a solution of n-BuLi (20 mmol, 8 ml, 2.5M in hexane) was added, stirred for 1 h and a neat solution of methyl iodide (5.68 g, 40 mmol) was added. The reaction mixture was allowed to warm to room temperature for overnight, quenched with aqueous saturated NH4Cl (100 ml) at room temperature. It was extracted with EtOAc (100 ml). The combined organic layer was washed with brine dried on Na2SO4, filtered and evaporated under reduced pressure to give a yellow oil which was crystallized from hexane to afford the product as a slightly yellow solid. (3.1 g, 81%).1H NMR (400 MHz, DMSO-d6): δ ppm 10.97 (s, 1H), 1.44 (s, 12H), 1.35-1.33 (m, 2H), 0.93-0.91 (m, 2H).
References:
US9527885,2016,B2 Location in patent:Page/Page column 617

74-88-4
346 suppliers
$15.00/10g

849022-29-3
10 suppliers
inquiry

24424-99-5
834 suppliers
$13.50/25G

849022-29-3
10 suppliers
inquiry