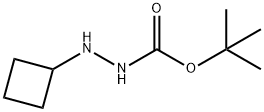
N'-cyclobutyl(tert-butoxy)carbohydrazide synthesis
- Product Name:N'-cyclobutyl(tert-butoxy)carbohydrazide
- CAS Number:959137-72-5
- Molecular formula:C9H18N2O2
- Molecular Weight:186.25
Yield:959137-72-5 25%
Reaction Conditions:
Stage #1: cyclobutanone;t-butoxycarbonylhydrazine in ethanol at 25; for 2.5 h;
Stage #2: with sodium cyanoborohydride;acetic acid in ethanol at 25; for 48 h;
Stage #3: with sodium hydrogencarbonate in ethanol;water; pH=8;
Steps:
41.a
Cyclobutanone (8.19 g, 116.85 mmol) was dissolved in ethanol (60 niL). tert-Buty carbazate (15.64 g, 118.34 mmol) was added. The reaction was stirred at 25 0C for 2.5 h before it was concentrated in vacuo. The residue was re-dissolved in ethanol (60 mL). Acetic acid (14.09 mL, 246.14 mmol) and sodium cyanoborohydride (15.46 g, 246.02 mmol) were added. The reaction was stirred at 25 0C for 48 h. The reaction was quenched via slow addition of saturated aqueous sodium bicarbonate solution (until pH 8 was reached). The resulting mixture was extracted with ethyl acetate (3 x 150 mL). The organic layers were combined, dried over magnesium sulfate, filtered and concentrated in vacuo to afford a yellow oil.
References:
WO2008/73982,2008,A2 Location in patent:Page/Page column 177-178
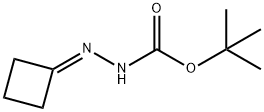
158001-20-8
21 suppliers
inquiry

959137-72-5
27 suppliers
inquiry
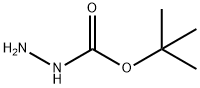
870-46-2
531 suppliers
$5.00/10g

959137-72-5
27 suppliers
inquiry
