
N-Cyclohex-3-enyl-4-methyl-benzenesulfonamide synthesis
- Product Name:N-Cyclohex-3-enyl-4-methyl-benzenesulfonamide
- CAS Number:210574-45-1
- Molecular formula:C14H17NO2
- Molecular Weight:231.29
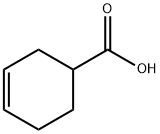
4771-80-6
267 suppliers
$5.00/10g

100-51-6
1379 suppliers
$5.00/100g

210574-45-1
1 suppliers
inquiry
Yield:210574-45-1 81%
Reaction Conditions:
Stage #1: 1,2,5,6-tetrahydrobenzoic acidwith diphenylphosphoranyl azide;triethylamine in toluene at 25 - 110; for 4 h;Inert atmosphere;
Stage #2: benzyl alcohol in toluene at 110; for 12 h;
Steps:
33.1 Step 1:
To a solution of cyclohex-3-enecarboxylic acid (20.0 g, 158.5 mmol) in toluene (360.0 mL) was added TEA (17.6 g, 174.3 mmol, 24.2 mL) and DPPA (45.8 g, 166.4 mmol, 36.0 mL). The mixture was degassed and purged with N2 for 3 times, it was stirred at 25°C for 1.5 h under N2 atmosphere. Then it was warmed to 1 10°C and stirred for another 2.5 h. BnOH (18.8 g, 174.3 mmol, 18.1 mL) was added to the mixture and the resulting mixture was stirred at 110°C for 12 h. The reaction was concentrated under reduced pressure to give a residue. The residue was purified by flash silica gel chromatography to afford benzyl cyclohex-3-en-l-ylcarbamate (33.0 g, 81.0% yield) as a white solid. MR (400MHz, CHLOROF ORM-d) δ 7.44 - 7.29 (m, 5H), 5.73 - 5.65 (m, 1H), 5.63 - 5.56 (m, 1H), 5.1 1 (s, 2H), 4.81 (br s, 1H), 3.88 (br s, 1H), 2.41 (br d, J= 17.2 Hz, 1H), 2.19 - 2.09 (m, 2H), 1.96 - 1.84 (m, 2H), 1.66 - 1.52 (m, 1H).
References:
WO2019/94641,2019,A1 Location in patent:Paragraph 00369

4771-80-6
267 suppliers
$5.00/10g

210574-45-1
1 suppliers
inquiry
2100-17-6
86 suppliers
$95.12/1g

210574-45-1
1 suppliers
inquiry
621-84-1
316 suppliers
$7.00/25g

210574-45-1
1 suppliers
inquiry

501-53-1
393 suppliers
$10.00/1g

210574-45-1
1 suppliers
inquiry