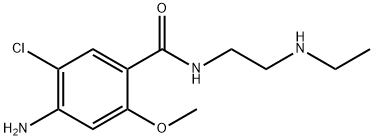
N-Desethyl MetoclopraMide synthesis
- Product Name:N-Desethyl MetoclopraMide
- CAS Number:27260-19-1
- Molecular formula:C12H18ClN3O2
- Molecular Weight:271.74

110-72-5
270 suppliers
$14.00/10g
7206-70-4
248 suppliers
$24.89/5g

27260-19-1
44 suppliers
$149.90/2 mg
Yield: 62%
Reaction Conditions:
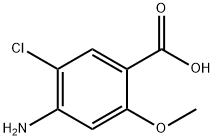
Stage #1:4-amino-5-chloro-2-methoxybenzoic acid with 4-methyl-morpholine;2-chloro-4,6-dimethoxy-1 ,3,5-triazine in N,N-dimethyl-formamide at 20; for 1 h;
Stage #2:N-ethylethane-1,2-diamine in N,N-dimethyl-formamide at 20; for 19 h;
Steps:
2 [0166] Synthesis of Ethylethylencdiamine-4-Amino-5-Chloro-2-Methoxybenzoic Acid Amide (Compound 6)
[0166] Synthesis of Ethylethylencdiamine-4-Amino-5-Chloro-2-Methoxybenzoic Acid Amide (Compound 6)[0167] To a DMF solution of 4-amino-5-choro-2-methoxybenzoic acid (1.8 g, ‘-8.9 mmol) and methylmorpholine (-2.7 g, --26.8 mmol), CDMT was added(---1 .6 g, --8.9 mmol) and the reaction mixture was stirred at room temperature for -1 hour. Thereafter, Nethylethlenediamine (-1.6 g, -17.9 mmol) was then added. The reaction mixture was stirred at room temperature overnight (-19 h), and HPLC indicated that the reaction was complete. DCM was added to the reaction mixture and the DCM solution was washed with O.IN NaOH/NaCl aqueous solution three times. After all solvents were removed, the product mixture was loaded on a silica gel column and eluted with DCM/Methanol in a Biotage. A white solid was obtained (--1.5 g, -5.5 mmol, -62% isolated yield). LC-MS: calc: 271.1; found: 271.1. Proton NMR also confirmed that it was the desired ethylethlenediamine-4- amino-5-chloro-2-methoxybenzoic acid amide (Compound 6).
References:
NEKTAR THERAPEUTICS;CHENG, Lin;RIGGS-SAUTHIER, Jennifer;ANAND, Neel, K. WO2014/43707, 2014, A1 Location in patent:Paragraph 0166; 0167

364-62-5
263 suppliers
$5.00/25mg

27260-19-1
44 suppliers
$149.90/2 mg

171367-22-9
66 suppliers
inquiry

27260-19-1
44 suppliers
$149.90/2 mg