
N-METHYL-D3-PIPERAZINE synthesis
- Product Name:N-METHYL-D3-PIPERAZINE
- CAS Number:1093380-08-5
- Molecular formula:C5H9D3N2
- Molecular Weight:103.18
110-85-0
554 suppliers
$5.00/5 g
865-50-9
135 suppliers
$25.00/1g
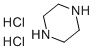
142-64-3
181 suppliers
$11.00/25g

1093380-08-5
25 suppliers
$498.60/5MG
Yield: 58.3%
Reaction Conditions:
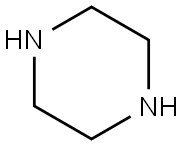
Stage #1:piperazine;piperazine dihydrochloride with water-d2 in ethanol for 1 h;Reflux;
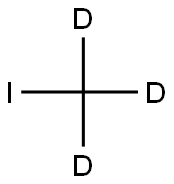
Stage #2:iodomethane-d3 in ethanol;water-d2 at 0 - 20;
Stage #3: with sodium hydroxide in ethanol;water-d2; pH=~ 9.0 at 0;
Steps:
2.1
EXAMPLE 2; 2-Methyl-4-(4-d3-methyl piperazin-1-yl)-10H-benzo[b]thieno[2,3-e][1,4]diazepine Step 1 1-Methyl-d3-piperizine: A mixture of piperizine (10.0 g, 116.1 mmol), piperizine dihydrochloride (18.45 g, 116 mmol), and ethanol/deuterium oxide (5:1, 60 mL) was heated at reflux for about 1 hour. The mixture was cooled to about 0° C., and d3-iodomethane (8.85 mL, 92.9 mmol) was added dropwise. The mixture was then stirred at ambient temperature for about 90 minutes. After cooling the mixture to about 0° C., the mixture was basified to a pH of about 9.0 with 2N sodium hydroxide. Standard extractive work up provided a crude residue, which was then purified by distillation at 120-130° C. to give the title compound as a colorless liquid (7.0 g, yield=58.3%). 1H NMR (400 MHz, CDCl3) δ 2.69-2.71 (m, 4H), 3.17-3.19 (m, 4H); IR (film) υ 3387, 3282, 2934, 2808, 2744, 2675, 2224, 2178, 2034, 1660, 1553, 1451, 1272, 1164, 836, 748 cm-1; MS 104 (M+1).
References:
AUSPEX PHARMACEUTICALS, INC. US2010/266711, 2010, A1 Location in patent:Page/Page column 15-16