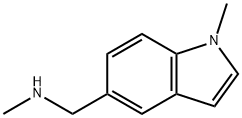
N-METHYL-N-[(1-METHYL-1H-INDOL-5-YL)METHYL]AMINE synthesis
- Product Name:N-METHYL-N-[(1-METHYL-1H-INDOL-5-YL)METHYL]AMINE
- CAS Number:709649-73-0
- Molecular formula:C11H14N2
- Molecular Weight:174.24

90923-75-4
63 suppliers
$50.00/250mg
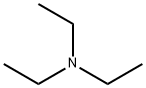
121-44-8
836 suppliers
$9.00/5g
![N-METHYL-N-[(1-METHYL-1H-INDOL-5-YL)METHYL]AMINE](/CAS/GIF/709649-73-0.gif)
709649-73-0
20 suppliers
inquiry
Yield:709649-73-0 45%
Reaction Conditions:
Stage #1: 1-methyl-1H-indole-5-carboxaldehyde;triethylamine in methanol; for 3 h;
Stage #2: with sodium tetrahydroborate in methanol at 20;
Stage #3: with sodium hydroxide;water
Steps:
47.a
a) Methyl-(l-methyl-lH-indol-5-ylmethyl)-amine; l-Methyl-lH-indole-5-carbaldehyde (338 mg, 2.13 mmol) was dissolved in anhydrous methanol (10 ml). Methylamine (0.80 ml of 33% solution in ethanol, 6.43 mmol) was added and the reaction was stirred for 3 h. The solution was concentrated to a brown oil and then dissolved in anhydrous methanol (10 ml). Sodium borohydride (83.0 mg, 2.19 mmol) was added and the mixture was stirred overnight at room temperature. Water (4 ml) was added and the solution was concentrated. Sodium hydroxide (8 ml, IN) was added and the aqueous layer was extracted with ethyl acetate (3x 20 ml). Combined organic layers were dried over MgSO4, filtered and concentrated to afford methyl-(l- methyl-lH-indol-5-ylmethyl)-amine (167 mg, 45%) as an orange oil: 1H ISlMR (400 MHz, DMSO-dg) δ 7.44 (s, IH), 7.34 (d, J= 8.0 Hz, IH), 7.261 (d, J= 3.2 Hz, IH), 7.11 (d, J=12.0 Hz, IH), 6.345 (d, J= 4.0 Hz5IH), 3.75 (s, 3H), 3.70 (s, 2H), 2.25 (s, 3H).
References:
WO2007/53131,2007,A2 Location in patent:Page/Page column 155