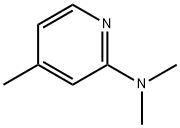
N,N,4-TRIMETHYL-2-PYRIDINAMINE synthesis
- Product Name:N,N,4-TRIMETHYL-2-PYRIDINAMINE
- CAS Number:20173-72-2
- Molecular formula:C8H12N2
- Molecular Weight:136.19
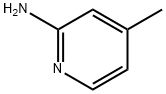
695-34-1
526 suppliers
$5.00/10g

64-19-7
1535 suppliers
$10.00/25ML

20173-72-2
17 suppliers
$45.00/50mg
Yield:20173-72-2 88%
Reaction Conditions:
with formaldehyd;sodium cyanoborohydride in water;acetonitrile at 0 - 20; for 168 h;
Steps:
1.1A
EXAMPLE 1; SYNTHESIS OF REAGENT [5-(7-CHLORO-2,5-DIMETHYL-PYRAZOLO[1,5-A]PYRIMΓDIN-3-YL)-4-METHYL-PYRIDIN-2- YL] -DIMETHYL- AMINE; EPO
References:
WO2006/44958,2006,A1 Location in patent:Page/Page column 38-39

695-34-1
526 suppliers
$5.00/10g

74-88-4
340 suppliers
$15.00/10g

20173-72-2
17 suppliers
$45.00/50mg

695-34-1
526 suppliers
$5.00/10g

50-00-0
843 suppliers
$10.00/25g

20173-72-2
17 suppliers
$45.00/50mg

695-34-1
526 suppliers
$5.00/10g

20173-72-2
17 suppliers
$45.00/50mg